Jim Palandri
Research Associate
Department of Earth Sciences
1272 University of Oregon
Eugene, Oregon 97403-1272
Jim might be found in:
- Ilya
Bindeman's stable isotope lab
- Elsewhere in Cascade or Columbia fixing something
- TSA's various shops -
the folks who can build almost anything
An ongoing project started in 2022 is restoration
of Gastherm.dat,
the thermodynamic database for GASWORK, SOLVGAS, and VOLCAL. These
volcanic gas modeling programs are analogous to programs Chim-XPT,
Solveq-XPT, and Geocal-XPT except that the mobile phase carrying the
chemical components is gaseous rather than aqueous. Related items
are here.
Handy links below, to mostly local reference info. Some *off-site
and so may be broken.
Items related to local applications Chim-XPT, Solveq-XPT, and
Geocal-XPT
- Soltherm.XPT
(zip)
Now with tourmaline and latest basis set component #66
univalent lithium. Sweet, sweet, lithium Based on
internally consistent mineral and gas phase data from Holland and
Powell 2011,
and updated Supcrt92 aqueous species data
- Soltherm_H2O-extended-H.xpt
A provisional version with the water enthalpy table extended from
600 to 1000C, and expanded to include steam and the low density
region outside 0.35 g/cc isochore, all down 1 bar. Allowing addition
of high T volcanic gas in fluid-fluid mixing calculations. The
temperature limit for the combined fluid remains 600°C.
- XPT-LogK
a utility for extracting and interpolating log K's in Soltherm
- Solprint-2011-XPT.txt
distilled from SOLTHERM as a user reference, showing balanced
reactions and log K's limited to liquid-vapor saturation from 25 to
350 °C
- SolthermBRGM.XPT
| SolprintBRGM.txt
*BRGM *Thermoddem data, reformatted
from the ToughReact *version.
For the Martians, and other low temperature peoples
- Gas.sto
gas stoichiometry data for program Geocal-XPT | Minox.dat
reactant stoichiometry data for Chim-XPT
- Manuals: Solveq-XPT
and Chim-XPT
- Fugacity coefficients in mixtures of H2O, CO2,
and NaCl at high P and T: program FUGCO,
with a worked example
as in the cited reference, and included as subroutines in Chim-XPT
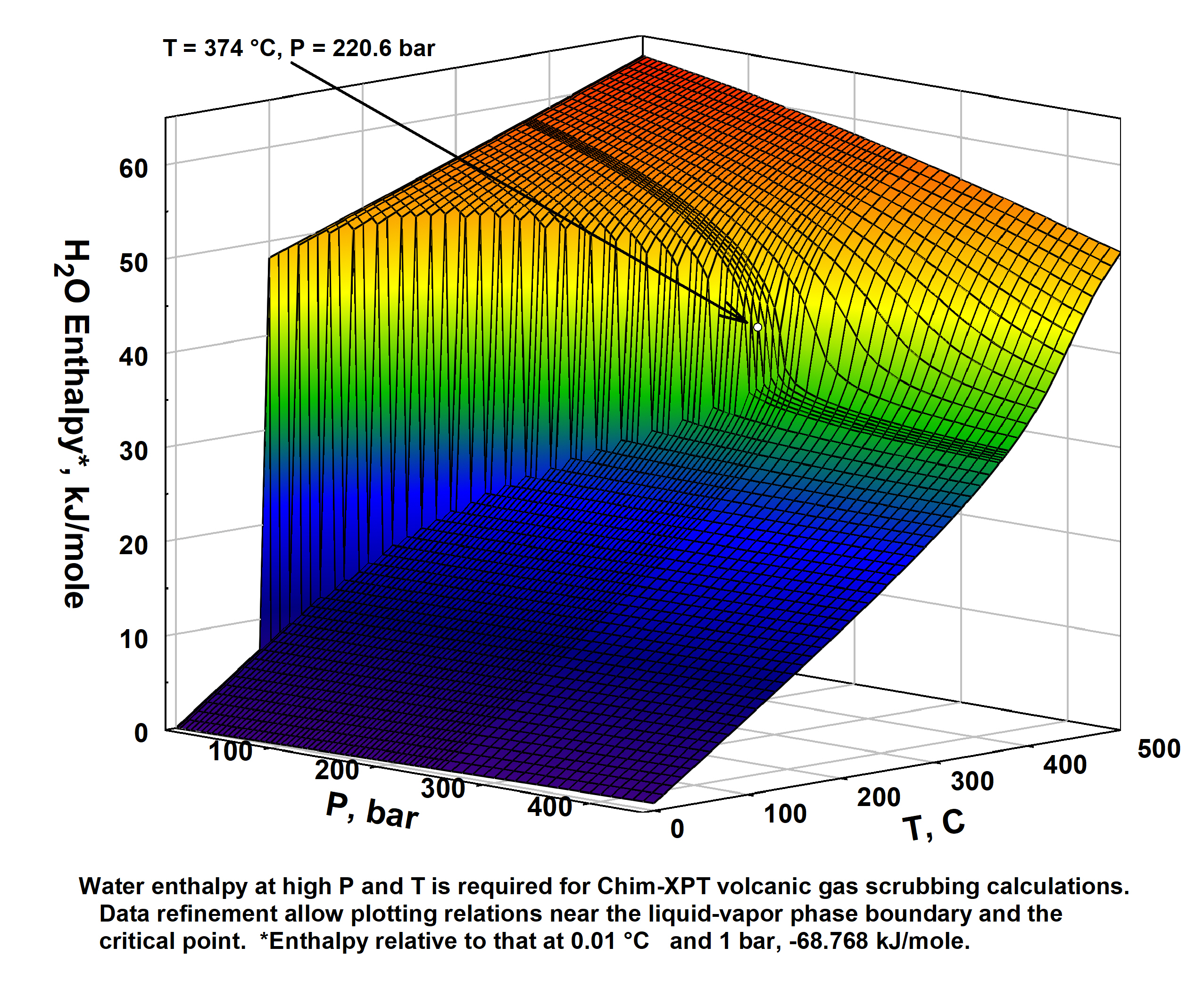
- Delicious water, what are thine properties?
So too ethereal vapor phase thereof - Steam Tables - pdf,
csv
Supcrt-related items for computing log K's in Soltherm
- Supcrt92.zip
the original distribution from Johnson et al. 1992
using Maier-Kelly heat capacity: Cp = a + b T + c T-2
- Supcrt96 | Source
| Win32
derived from Supcrt92 to use Maier-Kelly heat capacity, and adding
Powell and Holland 1990:
Cp = a + b T + c T-2 + d T-0.5
- Cprons96 | Source
| Win32
derived from Cprons92. Converts any (including any of those for
Supcrt92) properly formatted sequential access database to direct
access, suitable for use with Supcrt96
Some items related to the data used by Supcrt
- *Thermocalc
for phase equilibria computations application and data from Tim
*Holland.
The primary application for which the Holland and Powell 2011
compiled their data
- The most current Supcrt92 data might be found at the Gitlab ENKI portal, in Everett
Shock's Geopig
workgroup space, file Slop16.dat.
This group has done phenomenal work in adding data for organic
compounds.
Some info about files SPRONS and DPRONS Sequential-, and Direct-access
PROperties of Natural Substances, as per
Johnson et al 1992.
The sequential access file is for data editing, and is then
processed by Cprons into the direct access version for increased
Supcrt execution speed.
- SPRONS92.DAT
The original masterpiece
- SpronsHP2011.dat
The current version contains aqueous species from Slop07.dat and SpronsAqs,
minerals and gases from SpronsH&P2011minerals,
from Slop07 those that are not already present in SpronsH&P2011,
and from Sprons96Misc.
- dpronshp.dat
- The above file piped through Cprons96 and ready to use with
Supcrt96
- SpronsH&P2011minerals.dat
Mineral and gas phase data derived from Holland and Powell 2011
in the format for which Supcr96 and Cprons96 were originally hacked
in 1996. The data is *updated
and revised occasionally by Holland and Powell; the 2011 version can
be found here
- Sprons96Misc.dat
Bonus mineral and gas phase data from other sources, also properly
formatted for Supcrt96
- SpronsAqs.dat
Bonus aqueous phase data too, properly formatted for any version
of Supcrt. In particular, H4SiO4,aq.
from Stefαnsson 2001;
rarely does so very much hinge upon so very little
- SpronsGases.dat
Shows the gases section extended to 758 items for the GASTHERM
project
- Locally there are two SPRONS variants, Slop16.dat
from Shock et al. with only added H4SiO4,aq.
from Stefαnsson, and SpronsHP2011.dat
with identical aqueous species, all of
SpronsH&P2011minerals.dat, and the other miscellaneous items
from Sprons96Misc.dat and SpronsAqs.dat
Miscellaneous links:
Experimental petrology
- CSPV North
LabVIEW v.2012sp1/WinXP
- CSPV South
LabVIEW v.2018/Win10-64
- Related items
Isotope ratio mass spectrometry
- Info on laser fluorination
and extraction of oxygen from earth materials, for d18O and d17O
isotopic analysis
- Reference and other info. Most to
help in putting Humpty together again, and in semblance of working
order
Fluid inclusions
- Some photos
of the setup, before it was boxed up
Just for fun
- Geologic map [A,
B,
L;
USGS 7.5' - Clarno,
Muddy
Ranch] of Muddy Ranch and vicinity, north of Currant and Muddy
Creeks, Wasco, Wheeler, and Jefferson Counties, Oregon (1993). Aka Washington
Family Ranch, and before that Rajneeshpuram
- Streetcar map
of Eugene, OR, 1907-1928 Exposed track is still be seen on Moss,
Columbia, and University Streets. Modified from a map
published in Eugene Weekly in 2008
- Some photographs
of the I-5 Whilamut
Passage Bridge replacement and related construction, near
milepost OR 192
- Related items
The data
- It wants to be free
Bringing us to 55 of 82
stable elements in the Soltherm basis set. The other items being
metastable acetate, oxalate, succinate, malonate, aqueous CH4,
H2, N2, and NH3, radioactive UO2+
and Pm+3, and alternate redox component O2 -
redox state is defined by either paired sulfate-sulfide or H2O-O2.
A legacy from the era of 16-bit operating systems, when use of
sulfide for an oxidizing system, or O2 for a reducing
system, might cause an array underflow or that was claimed to be
the case but will never be verified. Since water must be present and
sulfate is so common, basis components sulfide and O2
were hard coded to be mutually exclusive. Pick one.
Bismuth-209 undergoes α-decay to 205Tl with
a half-life of 2.01x1019 years, or, ~1.46x1009
times the 1.38*1010 or so year age of our observable
universe. To not be a pedant but only in this one case, bismuth is
deemed stable. So 82 rather than 81.
Drumknott!
- Vetinari