



Russell J. Donnelly
541-346-4226 (Tel)
541-346-5861 (Fax)
![]()
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |
![]()
![]() The
Observed Properties of Liquid Helium
The
Observed Properties of Liquid Helium
![]() at the Saturated Vapor
Pressure
at the Saturated Vapor
Pressure
Chapter 8. Entropy
Adopted Database
Author(s) |
Key # |
Method |
Range (K) |
| Singsass Table 1 | 1 | Fountain Pressure | 1.59
|
| Van den Meijdenberg et al. | 2 | Fountain Pressure | 1.15
|
| Singsass Table 4 | 3 | Fountain Pressure | 2.15
|
| Singsass Table 2 | 4 | Fountain Pressure | 1.6
|
| Singsass Table 5 | 5 | Fountain Pressure | (T |
Comments and Key to Authors
1,3,4,5)
Singsass and Ahlers; Tables from Singsass Thesis, Ref. 7.
2) Ref. 4.
6) The spline has been adjusted to agree approximately with Singsass and
Ahlers at the lambda point. The spline returns =1.579 J/g.K and =3.025
J/g.K2.
7) Uncertainties: Singsass and Ahlers quote 0.1% precision, 0.5% accuracy.
Van den Meijdenberg et al. quote 1% precision, 3% accuracy.
8) Following the fountain pressure entropy spline are the knots and coefficients
of the fit to entropy data integrated from the recommended values of heat
capacity by
![]()
![]()
This
spline fit provides entropy data over both helium I and helium II regions.
9) Most heat capacity measurements are reported in Joules/mol.K. Entropy,
however is usually quoted in gram units. To convert data from J/g.K to
J/mol.K multiply by 4.0026. To convert from J/g.K to J/kg.K multiply by
1000.
Table 8.1. Adopted database for the fountain pressure entropy of liquid 4He.
T90 (K) |
Cs (J/mol·K) |
Key |
T90 |
u1 (m/s) |
Key |
| 1.15302927 | 3.950000E-2 | 2 | 1.90415059 | 7.320000E-1 | 2 |
| 1.20326685 | 5.150000E-2 | 2 | 1.90415059 | 7.356230E-1 | 4 |
| 1.25320267 | 6.650000E-2 | 2 | 1.95418895 | 8.420000E-1 | 2 |
| 1.30332399 | 8.550000E-2 | 2 | 1.95418895 | 8.472770E-1 | 4 |
| 1.35344288 | 1.075000E-1 | 2 | 2.00303241 | 9.663170E-1 | 1 |
| 1.40351505 | 1.325000E-1 | 2 | 2.00423358 | 9.630000E-1 | 2 |
| 1.45355661 | 1.620000E-1 | 2 | 2.00423358 | 9.711100E-1 | 4 |
| 1.50359791 | 1.960000E-1 | 2 | 2.05429341 | 1.105000E+0 | 2 |
| 1.55363712 | 2.370000E-1 | 2 | 2.05429341 | 1.113220E+0 | 4 |
| 1.59708138 | 2.812150E-1 | 1 | 2.10389749 | 1.271440E+0 | 1 |
| 1.60369037 | 2.840000E-1 | 2 | 2.10439892 | 1.275540E+0 | 4 |
| 1.60369037 | 2.900930E-1 | 4 | 2.15456275 | 1.468060E+0 | 4 |
| 1.65377936 | 3.370000E-1 | 2 | 2.15466305 | 1.469430E+0 | 1 |
| 1.65377936 | 3.425750E-1 | 4 | 2.15469110 | 1.469300E+0 | 3 |
| 1.69937308 | 3.977850E-1 | 1 | 2.16429053 | 1.512190E+0 | 1 |
| 1.70388228 | 3.980000E-1 | 2 | 2.16429053 | 1.513560E+0 | 4 |
| 1.70388228 | 4.034210E-1 | 4 | 2.16429267 | 1.511520E+0 | 3 |
| 1.75398060 | 4.660000E-1 | 2 | 2.16970354 | 1.540000E+0 | 3 |
| 1.75408079 | 4.725490E-1 | 4 | 2.16970398 | 1.540690E+0 | 1 |
| 1.80106080 | 5.449310E-1 | 1 | 2.17290000 | 1.557640E+0 | 1 |
| 1.80406509 | 5.450000E-1 | 2 | 2.17290343 | 1.557990E+0 | 3 |
| 1.80406509 | 5.502870E-1 | 4 | 2.17460000 | 1.570540E+0 | 1 |
| 1.85411884 | 6.330000E-1 | 2 | 2.17510000 | 1.571870E+0 | 1 |
| 1.85411884 | 6.381940E-1 | 4 | 2.17530000 | 1.573910E+0 | 1 |
| 1.90214931 | 7.301360E-1 | 1 | 2.17680000 | 1.578970E+0 | 5 |
![]()
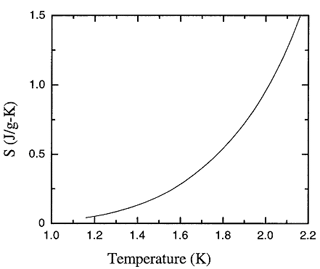
Figure 8.1. The recommended values of the fountain pressure entropy of liquid 4He as a function of temperature at saturated vapor pressure.
Table 8.2. Knots and coefficients for the spline fit of the fountain pressure entropy.
Knots |
Coefficients |
| K(1) = 1.153029 | C(1) = 3.949990E-2 |
| K(2) = 1.153029 | C(2) = 5.660103E-2 |
| K(3) = 1.153029 | C(3) = 9.933250E-2 |
| K(4) = 1.153029 | C(4) = 2.321544E-1 |
| K(5) = 1.402666 | C(5) = 5.183673E-1 |
| K(6) = 1.501863 | C(6) = 9.994261E-1 |
| K(7) = 1.801948 | C(7) = 1.506163E+0 |
| K(8) = 2.154592 | C(8) = 1.549628E+0 |
| K(9) = 2.164268 | C(9) = 1.575018E+0 |
| K(10) = 2.172874 | C(10) = 1.578977E+0 |
| K(11) = 2.176800 | |
| K(12) = 2.176800 | |
| K(13) = 2.176800 | |
| K(14) = 2.176800 |
![]()
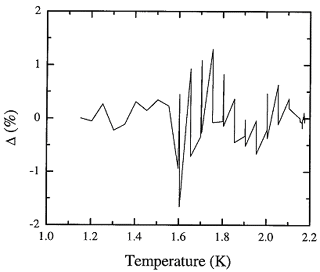
Figure 8.2. The fractional deviation of values of the adopted database from the recommended values for the fountain pressure entropy of liquid 4He expressed in percent.
Table 8.3. Recommended values of the fountain pressure entropy of liquid 4He.
T90 (K) |
S (J/g K) |
| 1.20 | 5.059E-2 |
| 1.25 | 6.560E-2 |
| 1.30 | 8.397E-2 |
| 1.35 | 1.057E-1 |
| 1.40 | 1.310E-1 |
| 1.45 | 1.600E-1 |
| 1.50 | 1.940E-1 |
| 1.55 | 2.343E-1 |
| 1.60 | 2.815E-1 |
| 1.65 | 3.357E-1 |
| 1.70 | 3.972E-1 |
| 1.75 | 4.662E-1 |
| 1.80 | 5.429E-1 |
| 1.85 | 6.279E-1 |
| 1.90 | 7.233E-1 |
| 1.95 | 8.319E-1 |
| 2.00 | 9.561E-1 |
| 2.05 | 1.099E+0 |
| 2.10 | 1.262E+0 |
| 2.15 | 1.450E+0 |
| 2.1761 | 1.577E+0 |
| 2.1762 | 1.577E+0 |
| 2.1763 | 1.577E+0 |
| 2.1764 | 1.578E+0 |
| 2.1765 | 1.578E+0 |
| 2.1766 | 1.578E+0 |
| 2.1767 | 1.579E+0 |
| 2.1768 | 1.579E+0 |
![]()
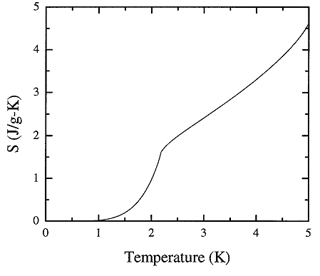
Figure 8.3. The recommended values of the entropy of liquid 4He obtained by integration of the heat capacity spline.
Table 8.4. Knots and coefficients for the spline fit of entropy integrated from the recommended values of heat capacity.
Knots |
Coefficients |
| K(1) = 0.10000 | C(1) = 6.60E-6 |
| K(2) = 0.10000 | C(2) = 7.60E-6 |
| K(3) = 0.10000 | C(3) = 1.60E-5 |
| K(4) = 0.10000 | C(4) = 4.61413E-5 |
| K(5) = 0.11123 | C(5) = 1.8400E-4 |
| K(6) = 0.21625 | C(6) = 4.68675E-4 |
| K(7) = 0.27672 | C(7) = 0.00121 |
| K(8) = 0.44817 | C(8) = 0.00236 |
| K(9) = 0.56369 | C(9) = 0.00561 |
| K(10) = 0.71969 | C(10) = 0.01978 |
| K(11) = 0.85360 | C(11) = 0.08337 |
| K(12) = 1.00186 | C(12) = 0.35824 |
| K(13) = 1.40184 | C(13) = 0.76882 |
| K(14) = 1.72912 | C(14) = 1.17803 |
| K(15) = 1.99887 | C(15) = 1.38629 |
| K(16) = 2.07775 | C(16) = 1.53613 |
| K(17) = 2.15384 | C(17) = 1.58271 |
| K(18) = 2.17680 | C(18) = 1.62419 |
| K(19) = 2.17680 | C(19) = 1.72261 |
| K(20) = 2.17680 | C(20) = 1.91862 |
| K(21) = 2.21160 | C(21) = 2.53529 |
| K(22) = 2.34820 | C(22) = 3.16601 |
| K(23) = 2.69075 | C(23) = 4.10379 |
| K(24) = 4.38277 | C(24) = 4.44077 |
| K(25) = 4.76361 | C(25) = 4.61570 |
| K(26) - K(29) | |
| = 5.000000 |
![]()
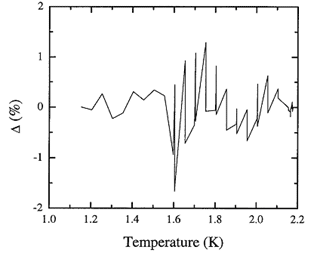
Figure 8.4. The fractional deviation of values of the adopted database from the recommended values for the entropy integrated from the heat capacity spline.
Table 8.5. Recommended values of entropy of liquid 4He obtained by integration of the heat capacity spline.
T90 (K) |
S (J/g K) |
T90 (K) |
S (J/g K) |
| 0.10 | 6.600E-6 | 2.25 | 1.720E+0 |
| 0.15 | 2.298E-5 | 2.30 | 1.783E+0 |
| 0.20 | 5.506E-5 | 2.35 | 1.838E+0 |
| 0.25 | 1.074E-4 | 2.40 | 1.890E+0 |
| 0.30 | 1.841E-4 | 2.45 | 1.939E+0 |
| 0.35 | 2.898E-4 | 2.50 | 1.985E+0 |
| 0.40 | 4.298E-4 | 2.55 | 2.030E+0 |
| 0.45 | 6.093E-4 | 2.60 | 2.074E+0 |
| 0.50 | 8.343E-4 | 2.65 | 2.116E+0 |
| 0.55 | 1.113E-3 | 2.70 | 2.158E+0 |
| 0.60 | 1.456E-3 | 2.75 | 2.200E+0 |
| 0.65 | 1.904E-3 | 2.80 | 2.242E+0 |
| 0.70 | 2.512E-3 | 2.85 | 2.284E+0 |
| 0.75 | 3.339E-3 | 2.90 | 2.326E+0 |
| 0.80 | 4.512E-3 | 2.95 | 2.367E+0 |
| 0.85 | 6.206E-3 | 3.00 | 2.409E+0 |
| 0.90 | 8.605E-3 | 3.05 | 2.451E+0 |
| 0.95 | 1.194E-2 | 3.10 | 2.492E+0 |
| 1.00 | 1.644E-2 | 3.15 | 2.534E+0 |
| 1.05 | 2.236E-2 | 3.20 | 2.576E+0 |
| 1.10 | 3.004E-2 | 3.25 | 2.619E+0 |
| 1.15 | 3.982E-2 | 3.30 | 2.661E+0 |
| 1.20 | 5.204E-2 | 3.35 | 2.704E+0 |
| 1.25 | 6.706E-2 | 3.40 | 2.747E+0 |
| 1.30 | 8.521E-2 | 3.45 | 2.790E+0 |
| 1.35 | 1.068E-1 | 3.50 | 2.834E+0 |
| 1.40 | 1.323E-1 | 3.55 | 2.878E+0 |
| 1.45 | 1.620E-1 | 3.60 | 2.923E+0 |
| 1.50 | 1.965E-1 | 3.70 | 3.014E+0 |
| 1.55 | 2.364E-1 | 3.75 | 3.060E+0 |
| 1.60 | 2.824E-1 | 3.80 | 3.107E+0 |
| 1.65 | 3.350E-1 | 3.85 | 3.154E+0 |
| 1.70 | 3.950E-1 | 3.90 | 3.203E+0 |
| 1.75 | 4.630E-1 | 3.95 | 3.251E+0 |
| 1.80 | 5.399E-1 | 4.00 | 3.301E+0 |
| 1.85 | 6.270E-1 | 4.05 | 3.351E+0 |
| 1.90 | 7.255E-1 | 4.10 | 3.402E+0 |
| 1.95 | 8.367E-1 | 4.15 | 3.454E+0 |
| 2.00 | 9.621E-1 | 4.20 | 3.507E+0 |
| 2.05 | 1.103E+0 | 4.25 | 3.561E+0 |
| 2.10 | 1.263E+0 | 4.30 | 3.616E+0 |
| 2.1760 | 1.578E+0 | 4.35 | 3.672E+0 |
| 2.1762 | 1.579E+0 | 4.40 | 3.728E+0 |
| 2.1763 | 1.580E+0 | 4.45 | 3.786E+0 |
| 2.1764 | 1.580E+0 | 4.50 | 3.846E+0 |
| 2.1765 | 1.581E+0 | 4.75 | 4.175E+0 |
| 2.1766 | 1.581E+0 | 4.80 | 4.250E+0 |
| 2.1767 | 1.582E+0 | 4.85 | 4.330E+0 |
| 2.1768 | 1.583E+0 | 4.90 | 4.416E+0 |
| 2.1769 | 1.583E+0 | 4.95 | 4.511E+0 |
| 2.1770 | 1.583E+0 | 5.00 | 4.616E+0 |
| 2.20 | 1.643E+0 |
Chronological Bibliography for Entropy
1 |
H. C. Kramers, J. D. Wasscher, and C. J. Gorter, "The Specific Heat of Liquid Helium Between 0.25 and 1.9 Degrees K," Physica 18, 329-337 (1952). |
| 2 | G. R. Hercus and J. Wilks, “The Specific Heat of Liquid Helium II as a Function of Pressure,” Philos. Mag. 45, 1163-1172 (1954). |
| 3 | O. V. Lounasmaa and E. Kojo, “The Specific Heat Cv of Liquid Helium Near the Lambda Curve at Various Densities,” Ann. Acad. Scien. Fennicae A VI, Physica 36, 1 (1959). |
| 4 | C. J. N. Van den Meijdenberg, K. W. Taconis, and R. De Bruyn Ouboter, “The Entropy of Helium II Under Pressure from Measurements on the Fountain Effect,” Physica 27, 197-218 (1961). |
| 5 | J. Wiebes, “Caloric Measurements on Liquid and Melting Helium Below 1.5 Kelvin,” Ph.D. Thesis, Kammerlingh Onnes Laboratory, 1969 (unpublished). |
| 6 | A. L. Singsaas and G. Ahlers, “Entropy of Helium II from 1.6 K to the Lambda Line,” Phys. Rev. B 29, 4951-4960 (1983). |
| 7 | A. L. Singsaas, “Entropy of Helium II and Universality Near the Superfluid Transition in 4He,” Ph.D. Thesis, University of California, 1984 (unpublished) |
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |

