A hydrogen atom has one electron. A helium atom has a heavier nucleus and two electrons. A lithium atom has a still heavier nucleus and three electrons. ... An oxygen atom has eight electrons. ...
An atom consists of a heavy nucleus surrounded by light
electrons.

A hydrogen atom has one electron. A helium atom has a heavier nucleus
and two electrons. A lithium atom has a still heavier nucleus and three
electrons. ... An oxygen atom has eight electrons. ...
A molecule consists of two or more atoms joined together.
Roughly speaking, one can think of the electrons as being in orbits
in an atom or molecule. But only certain orbits are allowed. For this
reason, the atom can have only certain energies. (Energies of atoms
are usually measured in
electron volts, abbreviated
eV.) The allowed energies
can be illustrated with a diagram:
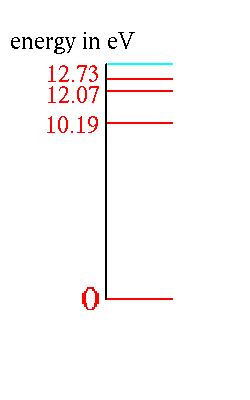 .
.
The example shown is for hydrogen, but each kind of atom or molecule
has its own energy level structure.
This picture shows the four lowest energy levels. There are more.
Energies above the blue line (~ 13.6 eV) are possible too. That corresponds to removing the electron from the hydrogen atom (ionizing the atom, making it an ion). The energies for an electron that isn't attached to the atom can be anything. Thus any energy above the ionization energy is allowed. For the moment we neglect this to keep the discussion simple.
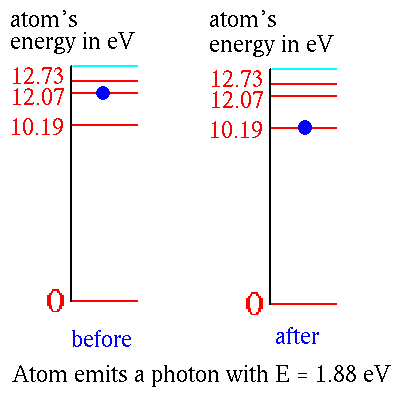
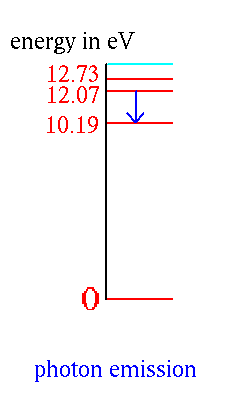
An atom in one of its "excited" levels can get to a lower level by
emitting a photon.
 .
.
The energy is carried away by the photon. Thus the energy of the photon is the difference
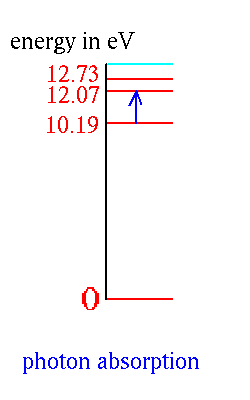
An atom in its ground state or in one of its excited states can absorb
a passing photon of the right energy and be raised to a higher energy
state:
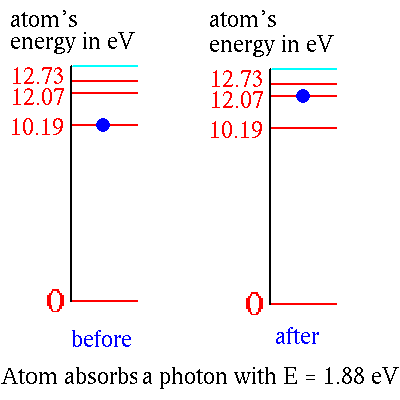
Note that the passing photon has to be just the right color or it can't
be absorbed.
We can use a simpler picture with arrows to illustrate these
processes:
But if you look at a thin, transparent gas, then you can see photons that come to you directly from an emitting atom.

Davison E. Soper, Institute of Theoretical Science, University of Oregon, Eugene OR 97403 USA soper@bovine.uoregon.edu