 |
Atomic Physics |
 |
Atomic Physics |
To understand why this is true, we discuss the structure of atoms. An atom can be represented as:
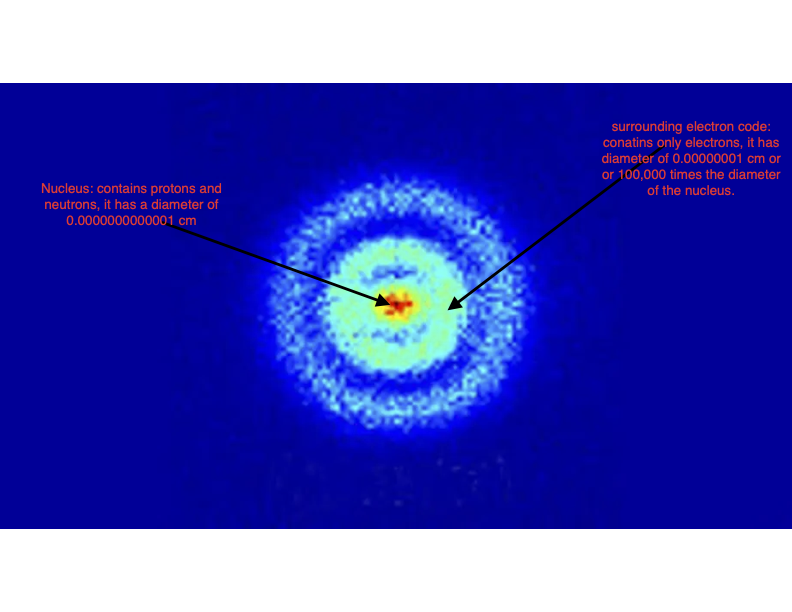
|
The neutrons and protons are packed into the nucleus of the atom which has a size of a few 10-15 m! The tiny unit 10-15 m is called a Fermi. Laid end-to-end, 4 trillion atomic nuclei would stretch over a distance of 1 inch!
The electrons are spread over a region of size 10-10 m! Huge compared to a nucleus but still miniscule. The tiny distance unit 10-10 m is called an Angstrom. This means that 100 million atoms laid end-to-end will stretch over a distance of 1 inch.
The element
to which a given atom corresponds is defined by the number
of protons it contains in its nucleus. The number of electrons and neutrons
is irrelevant in terms of the determination of the type of chemical element.
The number of protons in an atom (nucleus) is given by the Z of the nucleus. We have
Element | Number of Protons in Nucleus, Z |
hydrogen |
1 |
helium |
2 |
lithium |
3 |
berylium |
4 |
boron |
5 |
carbon |
6 |
nitrogen |
7 |
oxygen |
8 |
and so on |
For the rest of the periodic table, see here.
The preceding are isotopes of hydrogen.
The mass of a proton is ~1,836 times that of an electron and neutrons and protons have roughly the same mass ===> the mass of an atom is contained primarily in its nucleus while the nucleus is only
If we make an analogy with the Solar System, we can imagine that the nucleus is the Sun, and the electrons are the planets. The electrical force plays the role of gravity. This analogy is useful, however, there are important differences between how our Solar System works and how an atom works.
|
Kepler Orrery V: Youtube link showing orrery based on Kepler dara-- 726 planetry systems disvcovered by Kepler mission (1815 planets/candidates). The Solar System is shown for a sense of scale (the planets are not to scale). The planets are found nearly arbitrary orbit sizes around their stars. This means that the planets move in orbits that have arbitrary energy.
|
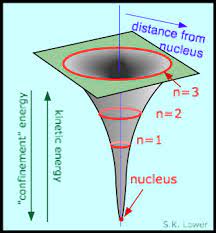
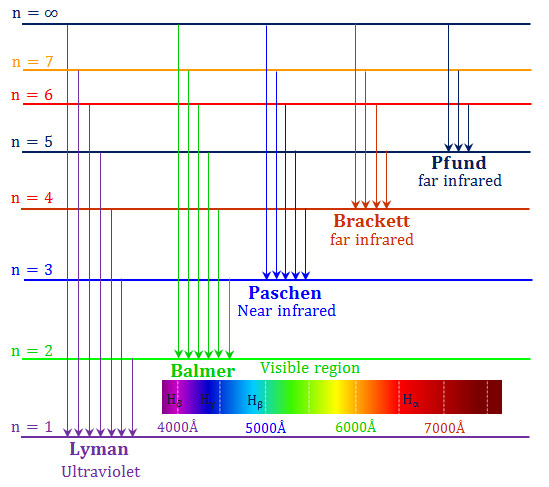
|
where n is the level. The lowest level is n = 1, the next higher level is n = 2, and so on.
Note that the largest transitions (longest arrows) require the highest energy photons, particles of light, because the transitions have the largest changes in energy. The photons which produce the largest transitions therefore involve the photons with the shortest wavelengths (since E = hc/W = hf).
Comment: (ii) To ionize an atom, all we
require is that the photon have enough energy to lift the electron
out of the well. If the photon has more than this threshold energy, it
simply gives the excess energy to the electron (as kinetic energy).
So, in terms of the appearance of the spectrum, we find that there is a
threshold where ionization begins followed by a broad trough which extends
to shorter wavelengths
(higher energies).
Such
ionization edges
are seen in the spectra of many stars.
Comment: (i) A neutral atom is denoted by I. An atom which has lost 1 electron is
denoted by II. An atom which has lost 2 electrons is denoted by III, and so on.
For example, neutral hydrogen is HI; singly ionized hydrogen is HII.
Some further definitions:
Lyman lines fall in the ultraviolet. Balmer lines fall in the optical. Paschen, Brackett, and Pfund lines fall in the infrared.
