Lectures 10&11
Igneous Rocks
Introduction
As we learned last time, melting of
the mantle usually produced basaltic magmas, compositions that are referred to as mafic because of their high proportions
of Mg and Fe. Mantle rocks
themselves are called ultramafic.
We
also learned that igneous rocks are commonly classifed by as intrusive or extrusive based on their ascent and cooling
history (and thus grain size – remember that slow cooling permits extensive
crystal growth, and thus intrusive rocks are completely crystalline, often with
fairly large crystals). Most of you are probably familiar with the extrusive
form of basaltic lava from exposure to the spectacular eruptive activity that
has occurred in Hawaii for the past twenty years [DVD examples].
Crystallization
of igneous rocks
Last week the simple Fa-Fo phase
diagram was introduced to illustrate several points about melting and the
composition of mantle melts. As
melting and crystallization are reversible processes, we can also use phase
diagrams to understand how melts crystallize [EX]. Some generalizations:
1. The
compositional paths followed by both the melt and the growing crystal depend on
the bulk (initial) composition of the melt.
2. The
composition of both the melt and the crystal at any point in the
crystallization process also depend on the amount of crystallization (the
extent to which the process has been completed).
3. For
minerals that exhibit solid solution (olivine, pyroxene, plagioclase),
crystallization proceeds in a continuous manner with the composition of the
mineral changing along with that of the liquid.
4. For
minerals that do not exhibit solid solution (because their crystal structures
are different), melting or crystallization will proceed in discrete (discontinuous)
steps [Ex: An-Di]. This means that
as a melt cools, these minerals will appear suddenly when the melt reaches the
appropriate temperature…
Bowen’s
reaction series [BRS]
The differences between minerals
that crystallize continuously and those that crystallize over discrete
temperature intervals was first described by the pioneer of igneous petrology,
NL Bowen. Bowen proposed a simple
explanation for the crystallization of magma in terms of common minerals that
exhibit one type of crystallization or the other … this simple scheme is now
known as Bowen’s Reaction Series:
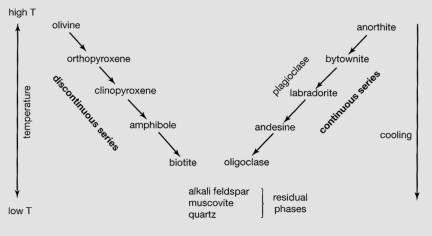
The
minerals on the discontinuous side of the series appear abruptly as the magma
cools. OR these minerals will also
start to melt abruptly in a solid rock as the rock is heated. The melting temperature is that of the
eutectic in the An-Di example.
In
contrast, the minerals on the continuous side shows the behavior of minerals
that crystallize as solid solutions, such as plagioclase [EX]. Note the following features:
•
minerals that crystallize (or melt) at high temperatures (olivine, pyroxene)
are rich in Mg and Fe and relatively poor in Si; igneous rocks that crystallize
these minerals will be mafic in composition (basalt; gabbro)
•
minerals that crystallize at the lowest temperatures (quartz, muscovite, alkali
feldspar) are rich in Si and Al; rocks that contain these minerals are felsic in composition (granite, rhyolite).
• rocks with intermediate minerals (amphibole, biotite) are
intermediate in composition (andesite, dacite, diorite).
Another
important observation is that minerals that crystallize at high temperatures
show less polymerization of the Si-tetrahedra than those that crystallize at
lower temperatures [compare BRS with Table 2.6 in the text].
Structure
of silicate liquids
The last observation above leads
directly to the subject of the structure of silicate liquids. Yes, these liquids do have structure,
which is created largely by varying degrees of bonding among SiO4
tetrahedra (polymerization). The
difference between a silicate liquid and a silicate mineral is that the mineral
has a definite structure that is the same throughout (what we refer to as
long-range order) while a silicate melt shows different types of polymerization
throughout the melt (short-range order).
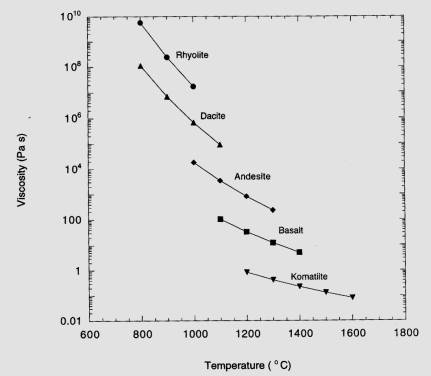
Additionally,
the degree of polymerization of the melt controls the viscosity (stickiness) of the melt. So, mafic magmas that have relatively
low Si contents have depolymerized melts that produce depolymerized crystals
(olivines with isolated tetrahedral and pyroxenes with single tetrahedral
chains); these melts tend to have a high temperature and a low viscosity (that
is, they are very fluid, as you have seen on the DVDs]. In contrast, felsic magmas have
abundant Si and Al, the melts are highly polymerized, they crystallize sheet
and framework silicates (mica, feldspar, quartz) and they are very viscous
(sticky). The consequences of
these characteristics are twofold:
1)
Basaltic magmas erupt easily; eruptions are dominated by fluid lava flows that
may travel 10s of kilometers.
2)
Rhyolitic magmas often cool and crystallize before reaching the surface, thus
forming the large granite intrusions that characterize the Sierra Nevada Mtns.
in CA (and the Wallowas). When
rhyolites do eruption, those eruptions are typically explosive because the gas
bubbles can’t escape from the melt.
Volatile
content of melts
Which
leads to another topic – volatiles.
Volatiles are elements that dissolve in magmas but transform to gas as
magma reaches the surface (and thus are depleted in lavas). Examples of important volatiles are H2O,
CO2, F, Cl, S (as H2S or SO2). Note that minerals high on BRS contain
no volatiles, while those toward the bottom (amphiboles and micas) contain
volatile elements. This tells us
that mafic magmas tend to be poor in volatiles (although not always), while
felsic magmas tend to be volatile-rich.
This is another reason why basaltic magmas tend to erupt passively while
felsic magmas tend to erupt more explosively.
Classification
of igneous rocks
Igneous rocks are classified on the
basis of (1) chemical composition and (2) texture.
Composition is controlled by
composition
of parent material
degree
of melting of parent material
modification
of composition by crystallization and differentiation
Compositional
ranges are shown in Box 5.1 of the text.
Note in particular variations in Mg & Fe (the mafic components) and
Si&Al (the felsic components).
Compositional classification may also be done by characteristic minerals
found in different rocks, as illustrated in Fig. 5.27 of your text.
Textures are controlled by conditions of
crystallization and cooling. By
texture we refer primarily to grain size of constituent crystals. Slowly cooled intrusive rocks tend to have
larger crystals than rapidly cooled extrusive (volcanic) rocks, which are
sometimes quenched so quickly that they are glassy (frozen liquid) rather than
crystalline. Combined textural and
chemical classifications and characteristics are given in Table 5.3 for the
felsic members, in Table 5.4 for the mafic members, and in Table 5.5 for the
ultramafic rocks. These
classification schemes are illustrated schematically in Fig. 5.28. Not shown here are typical
temperature and viscosity ranges, which I show below.