EUGENE, Ore. -- (Jan. 9, 2012) -- Much of what living cells do is carried out by "molecular machines" -- complexes of specialized proteins working together to carry out some biological function. How the minute steps of evolution produced these constructions has long puzzled scientists and provided a favorite target for creationists.
 In a study placed online ahead of regular publication Jan. 18 in the journal Nature, a team of scientists demonstrate how just a few small, high-probability mutations increased the complexity of a molecular machine more than 800 million years ago.
In a study placed online ahead of regular publication Jan. 18 in the journal Nature, a team of scientists demonstrate how just a few small, high-probability mutations increased the complexity of a molecular machine more than 800 million years ago.
By biochemically resurrecting ancient genes and testing their functions in modern organisms, the researchers showed that a new component was incorporated into the machine due to selective losses of function rather than the sudden appearance of new capabilities.
"Our strategy was to use 'molecular time travel' to reconstruct and experimentally characterize all the proteins in this molecular machine just before and after it increased in complexity," said senior author Joseph W. Thornton, professor of biology and member of the Institute of Ecology and Evolution at the University of Oregon. Thornton also is an early career scientist of the Howard Hughes Medical Institute and a professor of human genetics and evolution & ecology at the University of Chicago.
"By reconstructing the machine's components as they existed in the deep past," he said, "we were able to establish exactly how each protein's function changed over time and identify the specific genetic mutations that caused the machine to become more elaborate."
 |
| Joe Thornton provides snapshot: Read about it |
The collaborative study -- which was initiated by Victor Hanson-Smith in Thornton's molecular evolution laboratory and Gregory Finnigan in the biochemistry research group of the UO's Tom Stevens, professor of chemistry and member of the Institute of Molecular Biology -- focused on a molecular complex called the V-ATPase proton pump, which helps maintain the proper acidity of compartments within the cell.
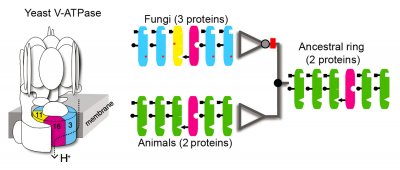
One of the pump's major components is a ring that transports hydrogen ions across membranes. In most species, the ring consists of six copies of two different proteins. However, in fungi a third type of protein has been incorporated into the complex.
To understand this increase in complexity, Thornton's group resurrected the ancestral versions of the ring proteins just before and just after the third subunit was incorporated. To do this, the researchers used a large cluster of computers to analyze the gene sequences of 139 modern-day ring proteins, tracing evolution backwards through time along the Tree of Life to identify the most likely ancestral sequences. Stevens' laboratory then used biochemical methods to synthesize those ancient genes, express them in modern yeast cells and determine their functions.
Thornton's group has helped to pioneer this molecular time-travel approach for single genes; this is the first time it has been applied to all the components in a molecular machine.
The researchers found that the third component of the ring in fungi originated when a gene coding for one of the subunits of the older two-protein ring was duplicated, and the daughter genes then diverged on their own evolutionary paths.
The pre-duplication ancestor turned out to be more versatile than either of its descendants: expressing the ancestral gene rescued modern yeast that otherwise failed to grow because either or both of the descendant ring protein genes had been deleted. In contrast, each resurrected gene from after the duplication could only compensate for the loss of a single ring protein gene.
The researchers concluded that the functions of the ancestral protein were partitioned among the duplicate copies, and the increase in complexity was due to complementary loss of ancestral functions rather than gaining new ones.
By engineering a set of ancestral proteins fused to each other in specific orientations, the group showed that the duplicated proteins lost their capacity to interact with some of the other ring proteins. While the pre-duplication ancestor could occupy five of the six possible positions within the ring, Thornton said, each duplicate gene lost the capacity to fill some of the slots occupied by the other, so both became obligate components for the complex to assemble and function.
"It's counterintuitive but simple," he said. "Complexity increased because protein functions were lost, not gained. Just as in society, complexity increases when individuals and institutions forget how to be generalists and come to depend on specialists with increasingly narrow capacities.”
The research team's last goal was to identify the specific genetic mutations that caused the post-duplication descendants to functionally degenerate. By reintroducing historical mutations that occurred after the duplication into the ancestral protein, they found that it took only a single mutation from each of the two lineages to destroy the same specific functions and trigger the requirement for a three-protein ring.
"The mechanisms for this increase in complexity are incredibly simple, common occurrences,” Thornton said. "Gene duplications happen frequently. It's easy for errors in copying to DNA to knock out a protein's ability to interact with certain partners. It's not as if evolution needed to happen upon some special combination of 100 mutations that created a complicated new function."
The accumulation of simple, degenerative changes over long periods of times could have created many of the complex molecular machines present in organisms today, he said. Such a mechanism argues against the intelligent-design concept of "irreducible complexity" -- that molecular machines are too complicated to have formed stepwise through evolution.
"I expect that when more studies like this are done, a similar dynamic will be observed for the evolution of many molecular complexes," he said.
“These really aren't like precision-engineered machines at all," Thornton added. "They are groups of molecules that happen to stick to each other, cobbled together during evolution by tinkering, degradation and good luck, and preserved because they helped our ancestors to survive."
Thornton, Stevens, Finnigan, a postdoctoral researcher, and Hanson-Smith, a graduate student in computer science, were co-authors on the Nature paper.
The National Institutes of Health, National Science Foundation and Howard Hughes Medical Institute funded the research. The NSF's Integrative Graduate Education and Research Traineeship Program also supports Hanson-Smith's research.
About the University of Oregon
The University of Oregon is among the 108 institutions chosen from 4,633 U.S. universities for top-tier designation of "Very High Research Activity" in the 2010 Carnegie Classification of Institutions of Higher Education. The UO also is one of two Pacific Northwest members of the Association of American Universities.
Media Contacts: Jim Barlow, director of science and research communications, 541-346-3481, jebarlow@uoregon.edu , or John Easton, University of Chicago, 773-795-5225, john.easton@uchospitals.edu
Source: Joseph W. Thornton, professor, University of Oregon, University of Chicago, (Currently at U-Chicago), 773-834-4323, joet1@uchicago.edu