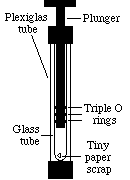
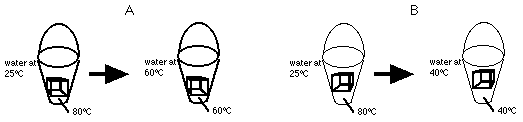8)
During this process, the internal energy of the nitrogen gas:
A. increases
B. decreases
C. is unaffected by the demonstration--has to be, the temperature doesn't change.
9) During this (second) process, heat energy:
A. is transferring from the fire syringe to the room.
B. is transferring from the room to the fire syringe.-- it had better if the gas is to stay at the same temperature.
C. transfer between the fire syringe and the room is negligible.
10) Two blocks, A and B, of equal mass, that are made of different materials, are each at a temperature of 80oC. Each of the blocks are immersed separately into a bucket, each bucket containing the same amount of water initially at room temperature.
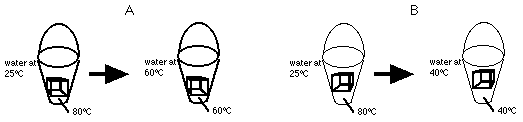
It is observed that the water with block A settles at a temperature of 60oC while the water with block B settles a temperature of 40oC.
What can you say about the properties of the materials of the blocks?
A. The material of A can store more energy than the material of B.
B. The material of B can store more energy than the material of A.
C. Energy is able to flow faster from block A than from block B.
D. Energy is able to flow faster from block B than from block A.
E. We cannot say anything unless we are given more information.
11) The notion of a "black body," where an object emits electromagnetic (EM) radiation according to its temperature, helps us:
A. explain how the warm air from a wood stove travels across the room to keep us warm.
B. estimate the temperature of the sun by examining the "color" of its light.
C. visualize how gas molecules create pressure by bouncing off walls.
D. to understand how signals are transmitted to radios and tv sets.
E. All of the above are correct.
12) Which of the following does NOT correctly describe a heat engine?
A. a heat engine involves the transfer of heat energy.
B. a heat engine converts some of the transferred heat energy to useful work.
C. heat engines operate in cycles such that the net heat energy transfer is always zero.
D. even perfect heat engine operate at less than 100% efficiency.
E. all of the above correctly describe a heat engine.
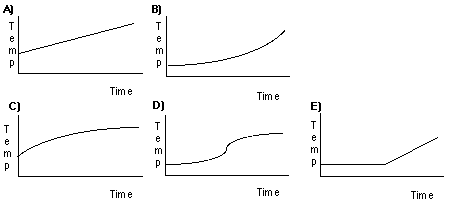
13) You have a covered Styrofoam cup which contains a mixture of ice and water at 0°C. The cup is perfectly insulated so that no heat can leak in or out. Heat energy is transferred to the mixture by an immersion heater (in the cup) at a steady rate. Which of the following graphs appropriately describes the shape of the temperature-time graph for this process? E) is the correct graph. The water remains at 0oC until all the ice is melted.
(14) To determine the temperatures of the two emitters yourself, you would look at the:
A. amounts of infra-red (IR) radiation under the curves.
B. wavelength where the curves have a peak power.
C. areas under the curves.
D. amount of visible EM radiation (light) under the curve.
A. to give weathermen something to talk about.
B. because the sun heats the Earth more at the equator.
C. because the Earth is spinning, which causes Coriolis forces on winds.
D. because nature abhors a vacuum.
E. because the Earth's axis of rotation is tilted relative to the plane containing the Earth, other planet and the sun (the ecliptic plane).