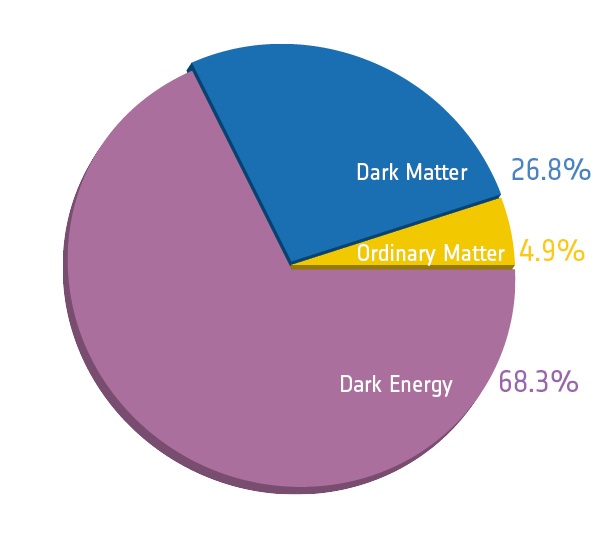
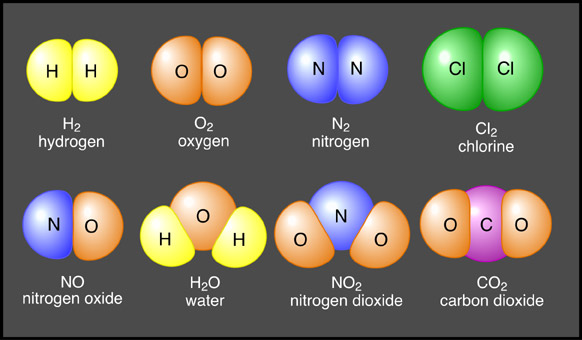
In solids, the positively charged nuclei (which are positive ions),
are forced to form a structure, many times a regular structure
like a lattice. The nuclei do not move about but they can vibrate about their
positions. The structures can stretch, compress, and twist a little,
but the general shapes do not change.
Electrons are free to move through the structure. Solids
tend to maintain their shape independent of the shape of the structure into
which they are placed. |
In liquids, the particles are free to move about but they cannot
move everywhere. For something like water, the volume occupied by the water
is roughly constant but liquid water can flow to assume a shape determined
by the shape of the container in which it is held.
|
In typical gases (vapors) the particles are neutral, the electrons
and nuclei are bound together and move as single units. The
neutral atoms (or molecules) are free to move
pretty much at will. The volume occupied by the gas is not fixed and
it is free to assume the shape of the container in which it is held.
|
In a plasma such as found in the Sun and stars,
the particles are similar to those in a gas
in that they are pretty much free to move at will. However, there is a
huge difference between a gas and a plasma in that in some atoms the
electrons have been stripped from the atoms and are free to move
in the mixture. These free electrons cause charge to flow giving rise
to electrical currents, electrical effects arise in plasmas.
This profoundly changes the ways
plasmas behave when compared to normal gases. Note that plasmas are
prevalent even on Earth because as pointed out in the next Chapter,
electrons also flow in some types of solids.
|