|
|

|
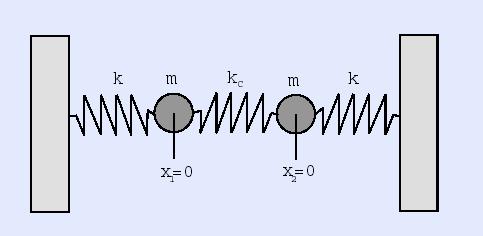
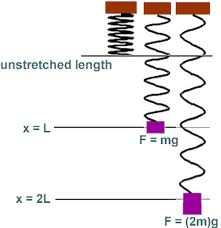
What makes things solids? Is it the chemical make-up of the object (the elements out of which it is composed) or is it something else? (We discuss this later in more detail when we talk about phase changes in the Heat and Thermodynamics secetion, but it is clearly not determined by the chemical make-up of the material as water exists as a solid (ice), a liquid (the oceans), and as a gas (steam).)Crystalline solids solid show regular structures where the atoms, molecules, nuclei, or ions are held rigidly in place, free only to vibrate about their locations. In amorphous solids, the atoms, molecules, nuclei, or ions are maintained in a structure but the structures can distort as the materials are able to flow (e.g., glass will flow); this motion is in addition to vibrations. In both cases, however, the particles interact through electrical forces where we know that the strength of the force falls off with distance. The particles thus try to arrange themselves so that the average electrical repulsion between them is minimized and so settle into distinct structures. In some solids, the electrons are able to migrate through the structure while in others, the electrons are bond more tightly to the individual sites in the structure. In the first case, heat and electrical charge (current) can flow easily through the solid while in the latter case, heat and electrical charge will not flow easily.
| 
|