|
Chapter 13: LiquidsHow do Liquids Compare to Solids?
We know what we mean when we say something is a solid. We recognize a bar of lead and a rock (olivine) are solids, and that soft drinks, the oceans, and rivers are liquids, and so on. In general terms, we recognize that solids are rigid and/or malleable structures while liquids can flow. (Recall the comment about amorphous solids in this case; Are amorphous solids, solids or liquids?)In this Chapter, we consider how liquids differ from solids, in a physical sense, and some properties of liquids in everyday life. In the top left we see two examples of water (the molecule composed of two hydrogen atoms and one oxygen atom, H2O). To the left, the bottom compares two forms of water. The right hand model shows the well-structured solid form of water, ice, and the left hand model shows a more disorgainized collection of water molecules, a liquid. Interestingly, water expands when it goes from liquid to solid and liquid water has a density of 1 gram per cubic centimeter (1 gram per sugar cube) while solid water (ice) has a density of 0.92 grams per cubic centimeter (0.92 grams per sugar cube)! Solid water is less dense than liquid water which, as we point out later, means that ice floats in liquid water (see the iceberg to the left). |
|

|







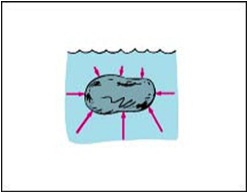









 Drop Formation
Drop Formation
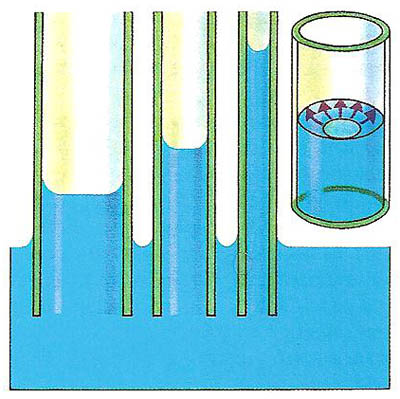
 Capillarity
Capillarity
 Menisci
Menisci