|
Chapter 14: Gases and PlasmasHow do Gases Compare to Liquids and Solids?
We know what we mean when we say something is a liquid or solid,
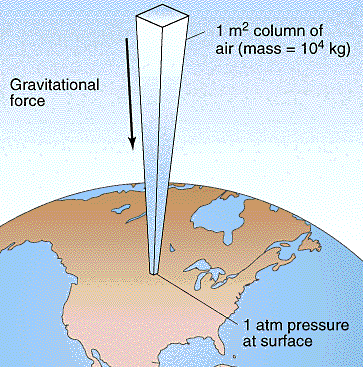
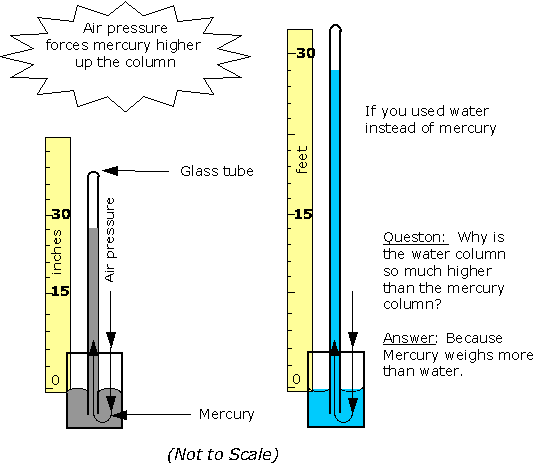
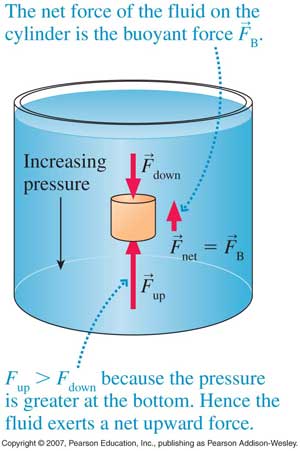
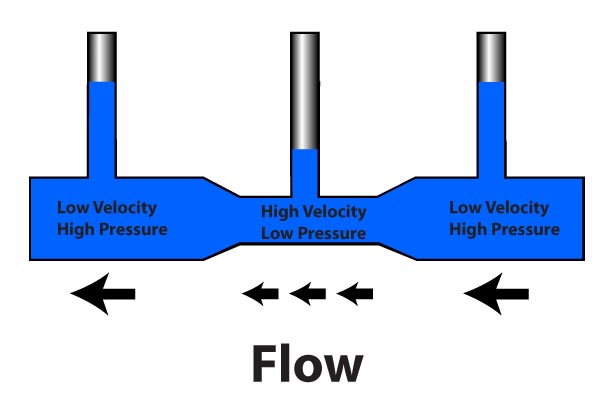
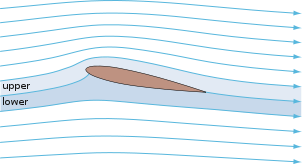
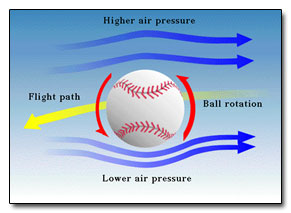
and so on. In general terms, we recognize that solids are rigid and/or malleable structures, liquids can flow but the atoms, molecules, or ions that make them up still interact and are sticky (cohesion). In this Chapter, we indicate how gases differ from solids but are similar to liquids in a certain sense, and some effects of gases in everyday life. The atmospheric pressure changes with height (NOAA) are at left. The pressure unit for this plot is millibarsis where 1,000 millibars is 1 bar or 1 atmosphere (1 atm). Note that there is no upper surface to the atmosphere; the atmosphere simply fades away as the gas rises as far as it can (due to the confinement of gravity). |
|
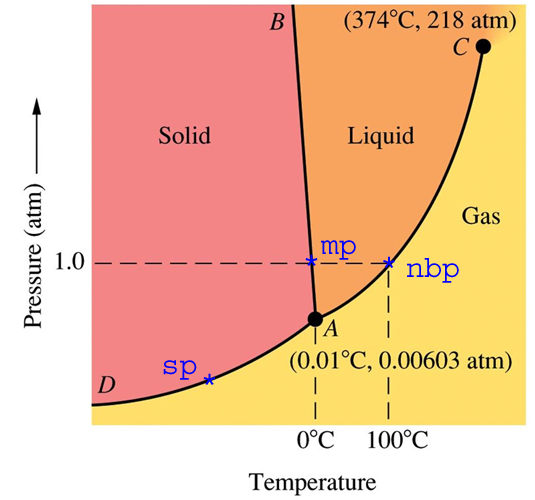 |