|
|

|
|
|
|
|
|
|
|


|
Hot dense objects like stars (and the Universe, see left) radiate in a manner similar to materials known as Blackbodies. Blackbodies are defined not by their emission properties but rather, by their ability to absorb electromangetic radiation. Above, we see objects coated with aluminum, silicon, and aluminum nitride, and an object coated with the blackest material yet made (reflects < 0.1 % of incident light).
Q: What is a better color to wear in sunny climates? in cold climates? The processes of absorption and emission are opposites (inverses) of each other. It turns out that good absorbers are also expected to be good emitters of radiation. This property allows us to calculate the emission properties of blackbodies. The theory for blackbody radiation was worked out in the 1800s by Planck. Planck's theory for these idealized objects capable of perfect absorption and so perfect emission led to the development of Quantum Mechanics and to one of the major physics revolutions in the early 1900s.
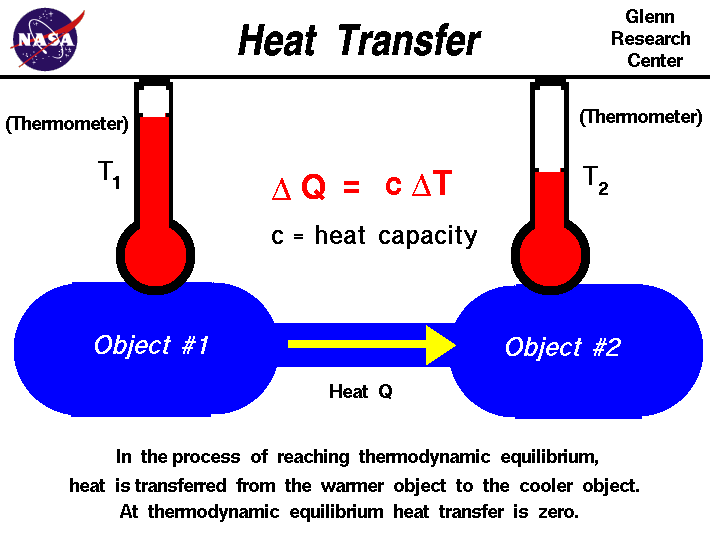
An object gains heat by absorbing radiation (H) and loses heat by emitting radiation (C). So, whether an object heats or cools is determined by which process wins.
The rate at which an object heats or cools is determined by
Q: Does a cup of hot black coffee cool at a faster
rate than a cup of hot coffee with milk?
Which cup takes longer to cool to room temperature?
Q: Does a bowl of hot water cool at a faster
rate than a bowl of cold water in the freezer of your refrigerator?
Which takes longer to freeze (to turn to ice)?
|
|

|
|


|
The Sun has surface temperature 5,527 degrees Celsius (5,800 Kelvin) while the surface temperature of the Earth is around 10-15 degrees Celsius (283 Kelvin).
Yes. The temperature of a hot, dense object
like the Sun and the surface of the Earth, controls the energy of the
electromagnetic radiation produced. We can estimate the radiation properties
of such objects because they resemble the idealized materials known as
Blackbodies, the perfect absorbers of radiation
mentioned above.
For blackbodies, we know that the hotter is a source of radiation,
the more energetic will be the electromagnetic radiation produced
(the higher the resultant frequency of the peak radiation produced).
The precise relationship for blackbodies and thus objects like the Sun
is given by
Wien's Law (shown in the Figure to the right of the Sun).
The Sun (temperature of around 5,700 Kelvin)
has peak power output in the visible band of the
electromagnetic spectrum,
according to Wien's Law. The temperature of the
Earth, on average, is 10-15 degrees Celsius (temperature of
280-290 Kelvin) and the Earth radiates
lower energy radiation at peak output than does the Sun.
The Earth radiates
Infrared radiation (IR)
according to Wien's Law.
What are the consequences of the different energies for Solar and Terrestrial radiation? |
|
 |
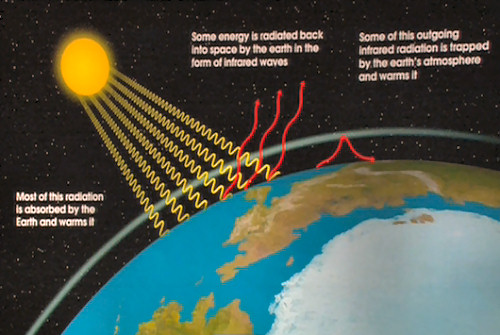
The atmosphere of the Earth is made up of 79 % nitrogen molecules, 21 % oxygen molecules with trace amounts of water vapor and carbon dioxide. The water vapor and carbon dioxide are both Greenhouse Gases. The materials in the atmosphere of the Earth allow visible light and parts of the UV from the Sun to pass through the atmosphere and to strike the surface of the Earth. This includes the water vapor and carbon dioxide. Because the temperature of the Earth is 10-20 degrees Celsius, the absorbed energy is re-radiated in the Infrared (IR) portion of the electromagnetic spectrum.This has the following consequences:
|