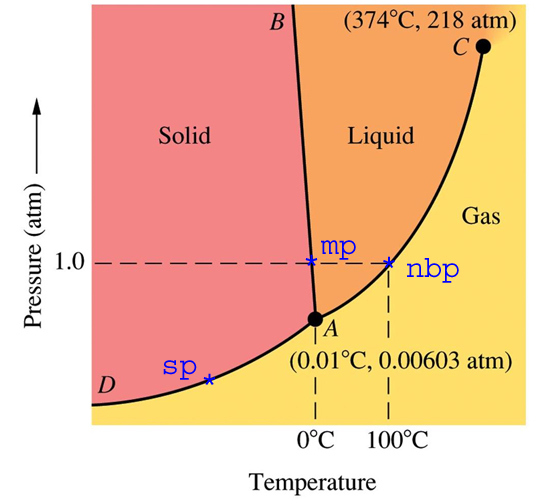
|
and the gas to solid phase change as deposition.
|

|
and the gas to solid phase change as deposition.
|
|
====> freezing |
|
====> condensation |
|
|
====> deposition |
|
|
Supercooled WaterThe phase transition from liquid water ---> solid water (ice), is interesting. If we cool water slowly, without jostling the water around, we can maintain the water in its liquid even if we drop its temperature below 0 degress Celsius. The is known as Supercooling. We demonstrate this in class. Also see supercooling Youtube video. There is an energy difference between the phases of water, it takes energy to turn ice into liquid water (it takes energy to enable the water molecules to move freely, they need to be set free). The energy difference between the phases will be abruptly released at the time of the transition from liquid water to ice (see the freezing water produces heat (Youtube video). |
Evaporation, Cooling, and SweatingThe phase transition from liquid water ---> gas, evaporation, is interesting in terms of its cooling effects.
 As you heat water, its temperature goes up which means that the average translational energy of the water molecules increases. Note that some molecules move slower than average and some move faster than average. When the temperature approaches 100 degrees Celsius, it is easiest for the fastest water molecules to escape ===> the average energy is dereased and the water is able to not only shed energy but to also cool. (What would happen if molecules of all energies managed to escape equally?) People have many sweat glands on their bodies and so are able to secrete water onto their skin. When the water evaporates it acts to cool. This way people can shed body heat and cool down after exertion. Interestingly, not all animals sweat and some that do, don't have extensive sweat glands:
|




|
Refrigerators

 A refrigerant is pumped through the above system extracting energy from the inside compartment of the refrigerator and shedding to air in the room. Refrigerants are materials with low melting and boiling points. Currently, a popular refrigerant is Freon R-22 (an HCFC, not an ozone depleter but a mild greenhouse gas). At 1 atmosphere, R22 has a boiling point of -40.7 degrees Celsius and a melting point of -160 degrees Celsius. In the past, Chloroflourocarbons (CFCs) were popular refrigerants, but it was discovered that CFCs damaged the ozone layer with long-lasting effects. CFCs released as the surface of the Earth, through leaky refrigerators and air conditioners, rise up to the stratosphere (where the ozone layer lives) after several years and then linger for decades in the stratorsphere destroying ozone). This is an unhappy circumstance as the ozone layer shields the surface from Solar UV radiation, and the heating of the stratosphere by the absorption of Solar UV radiation effectively traps the Earth's water vapor near the surface of the Earth in the troposhere. Without the ozone layer, we would be exposed to more Solar UV and we would slowly lose our water. Today, we use more environmentally safe refrigerants.
|
Outside of the refrigerator, the compressor compresses refrigerant gas, increasing its pressure. As the pressure increases, so does its temperature. This high-pressure, high-temperature gas enters the coil on the outside of the refrigerator. Heat flows from the high-temperature gas to the lower-temperature room air surrounding the coil. This heat loss causes the high-pressure gas to condense to liquid giving off heat to the air in the room behind the refrigerator.Next, the liquid refrigerant in the external coil drops in pressure as it passes through an expansion valve into a coil inside the insulated compartment of the refrigerator. The low-pressure liquid refrigerant is lower in temperature (cooler) than the air inside the refrigerator and heat is then transferred from the air inside the refrigerator to the liquid refrigerant. This causes the temperature inside the refrigerator to decrease. The absorbed heat causes the liquid refrigerant to start to boil. When all of the refrigerant changes to gas, the cycle repeats.  Heat pumps are essentially the same as this cooling cycle where instead of extracting energy to lower the temperature in the refrigerator, warm water is injected into the system (to replace the cold air in the refrigerator). This warm water heats the refrigerant which then boils, is run through the compressor is compressed and heated. It flows through the condensation coils where it sheds energy to the room, cools and liquefies, and the cycle repeated. |

|
|
 |