Update 2:
The Sample Analysis at Mars (SAM) instrument on Curiosity made
the first definitive detection of organic molecules at Mars. Organic molecules
are the building blocks of all known forms of terrestrial life, and consist
of molecules made primarily of carbon, hydrogen, and oxygen. Organic molecules
can also be made by chemical reactions which don't involve life, and it is
not known if the material found came from ancient Martian life or from a
nonbiological process such as chemical reactions in water in ancient
Martian hot springs or organic material brought to Mars by interplanetary
dust or fragments of asteroids and comets.
Curiosity with its suite of instruments including SAM was sent to
Mars in 2011 to discover more about the ancient habitable Martian environment
by examining clues in the chemistry of rocks and the atmosphere.
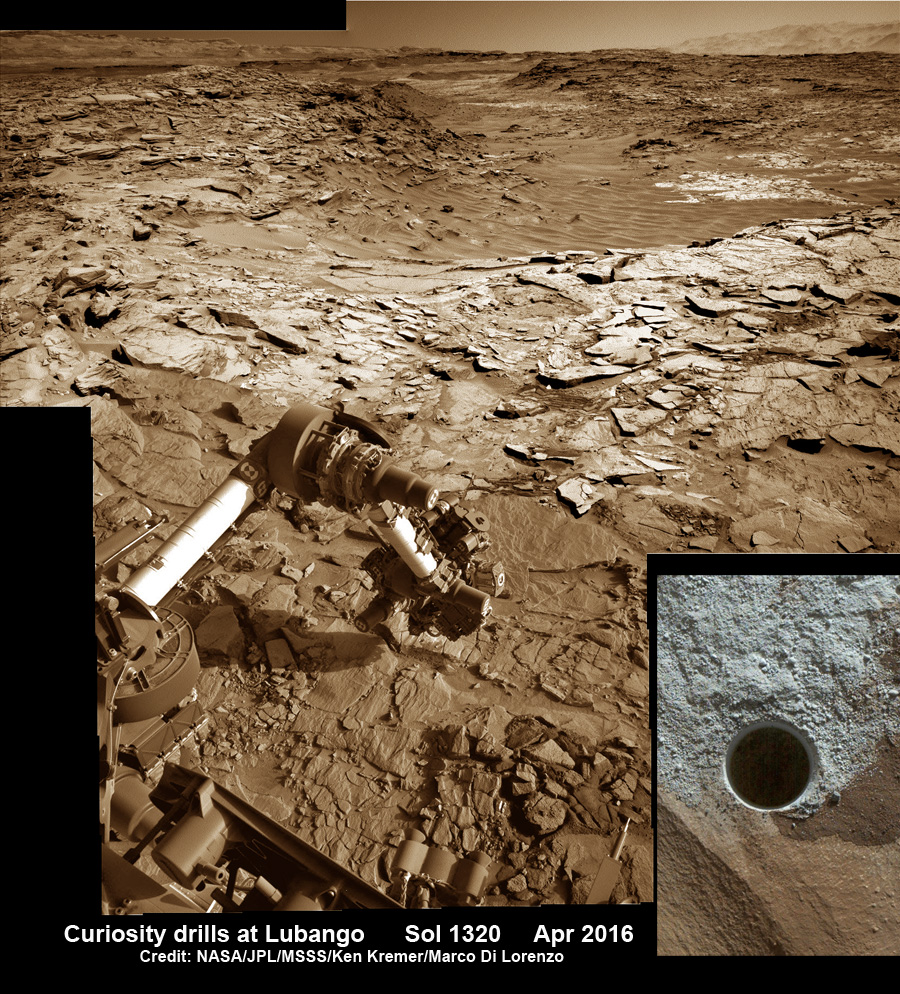
Organic molecules were found in a drilled sample of the Sheepbed mudstone
in Gale crater, the landing site for Curiosity.
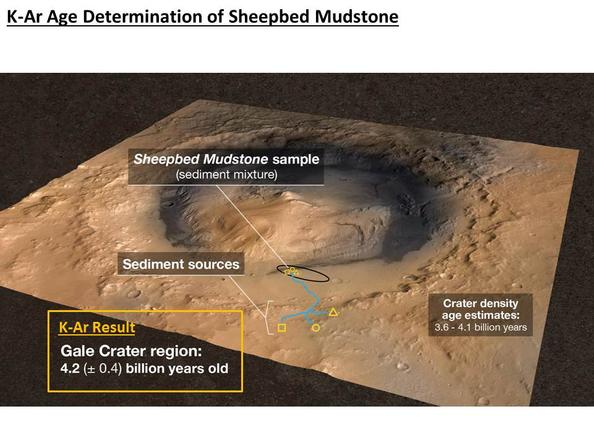
The crater is thought to
be the site of a lake billions of years ago, and rocks like mudstone formed
from sediment in the lake. the mudstone was found to contain 20% smectite
clays. On Earth, smectities are provide high surface area and optimal
interlayer sites for concentration and preservation of organic compounds
when rapidly deposited under reducing chemical conditions.

The discovery shows that the ancient environment of Mars offered a supply of
reduced organic molecules for use as building blocks for life and an energy
source for life. Other analysis of the same mudstone revealed that water and
chemical elements essential for life and a different chemical energy
source.
Life on Earth started ~3.8 billion years ago. There are sites on Mars that
had the same conditions at that time; liquid water, a warm environment, and
organic matter.
The organic molecules also contained chlorine atoms, and include
chlorobenzene and several dichloroalkanes, such as dichloroethane,
dichloropropane and dichlorobutane. Chlorobenzene is the most abundant with
concentrations between 150 and 300 parts-per-billion. Chlorobenzene is not a
naturally occurring compound on Earth. It is used in the manufacturing process
for pesticides (insecticide DDT), herbicides, adhesives, paints and rubber.
Dichloropropane is used as an industrial solvent to make paint strippers,
varnishes and furniture finish removers, and is classified as a carcinogen.
It's possible that these chlorine-containing organic molecules were present as
such in the mudstone. However, according to the team, it's more likely that a
different suite of precursor organic molecules was in the mudstone, and that
the chlorinated organics formed from reactions inside the SAM instrument as
the sample was heated for analysis. Perchlorates (a chlorine atom bound to
four oxygen atoms) are abundant on the surface of Mars. It's possible that as
the sample was heated, chlorine from perchlorate combined with fragments from
precursor organic molecules in the mudstone to produce the chlorinated
organic molecules detected by SAM.
In 1976, the Gas Chromatograph Mass Spectrometer instrument on NASA's Viking
landers detected two simple chlorinated hydrocarbons after heating Martian
soils for analysis (chloromethane and dichloromethane). However they were
not able to rule out that the compounds were derived from the instrument
itself, according to the team. While sources within the SAM instrument also
produce chlorinated hydrocarbons, they don't make more than 22
parts-per-billion of chlorobenzene, far below the amounts detected in the
mudstone sample, giving the team confidence that organic molecules really
are present on Mars.
|








