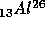 is not stable.
(Oh,
wait, the 13 says that the Aluminum nucleus contains 13 protons and the
26 says that the Aluminum nucleus contains 26 protons and neutrons, that
is,
the nucleus contains 26 - 13 = 13 neutrons.) The Aluminum decays as
is not stable.
(Oh,
wait, the 13 says that the Aluminum nucleus contains 13 protons and the
26 says that the Aluminum nucleus contains 26 protons and neutrons, that
is,
the nucleus contains 26 - 13 = 13 neutrons.) The Aluminum decays as
Some atomic nuclei are not stable. If they are allowed to sit,
they will spontaneously break apart into smaller nuclei releasing
particles
and energy. For example, the isotope
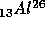 is not stable.
(Oh,
wait, the 13 says that the Aluminum nucleus contains 13 protons and the
26 says that the Aluminum nucleus contains 26 protons and neutrons, that
is,
the nucleus contains 26 - 13 = 13 neutrons.) The Aluminum decays as
is not stable.
(Oh,
wait, the 13 says that the Aluminum nucleus contains 13 protons and the
26 says that the Aluminum nucleus contains 26 protons and neutrons, that
is,
the nucleus contains 26 - 13 = 13 neutrons.) The Aluminum decays as
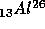 breaks up into
breaks up into
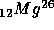 + positron + energy
+ positron + energy
This process has a half-life of
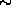 720,000 years. The
half-life
is the amount of time over which the probability is 1/2 that the nucleus
will
decay. Some important short half-life radioactive nuclei are
720,000 years. The
half-life
is the amount of time over which the probability is 1/2 that the nucleus
will
decay. Some important short half-life radioactive nuclei are
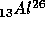 to
to
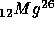 with
t = 0.72 M years
with
t = 0.72 M years
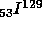 to
to
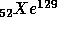 with
t = 16 M years
with
t = 16 M years
Some important long half-life radioactive nuclei are
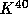 to
to
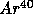 ,
,
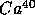 with
t = 1.25 B years
with
t = 1.25 B years
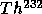 to
to
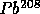 with
t = 14 B years
with
t = 14 B years
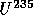 to
to
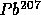 with
t = 0.7 B years
with
t = 0.7 B years
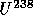 to
to
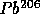 with
t = 4.5 B years
with
t = 4.5 B years
The shorter half-life nuclei add to the initial heating of the planets while the longer half-life nuclei add to the current heating of the interiors of the planets (the interiors of some of the Terrestrial planets are still quite hot.