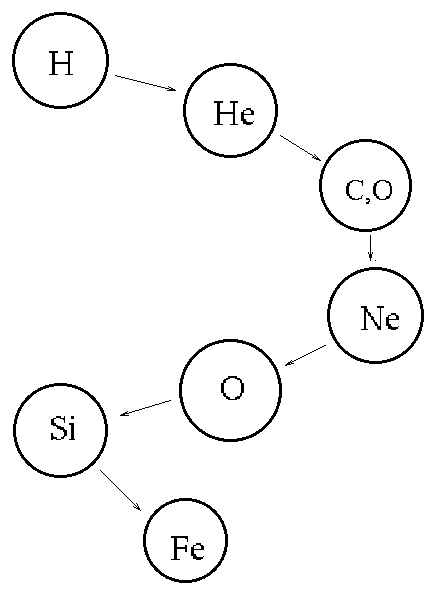
| The process starts with the simplest element hydrogen (1 proton) and produces more and more complex elements until the chain stops with iron (26 protons). Fe is the most stable nuclear structure (is the easiest nucleus to hold together). Thus, when combining elements up to Fe a little bit less energy is required to hold the product nucleus together than was required to hold the individual fusing nuclei together. This is the energy released in the fusion reaction. If you try to fuse elements more massive than Fe, then the product nucleus requires more energy to hold it together than was contained in the individual fusing nuclei. In this case, you must add energy to make the fusion process go! This is not an efficient way to generate energy. |
