Nearby Supernova and Earth
Nearby supernovas are rare. There have only been a
handful "observed" in our
Galaxy over the last 1,000 or so years.
The best guess as to the supernova rate
is 1 per 40 (± 10) years in galaxies similar to ours
(based on studies of other galaxies). Around 85 % of the supernovas
observed are core-collapse supernovas, Type II supernovas.
 |
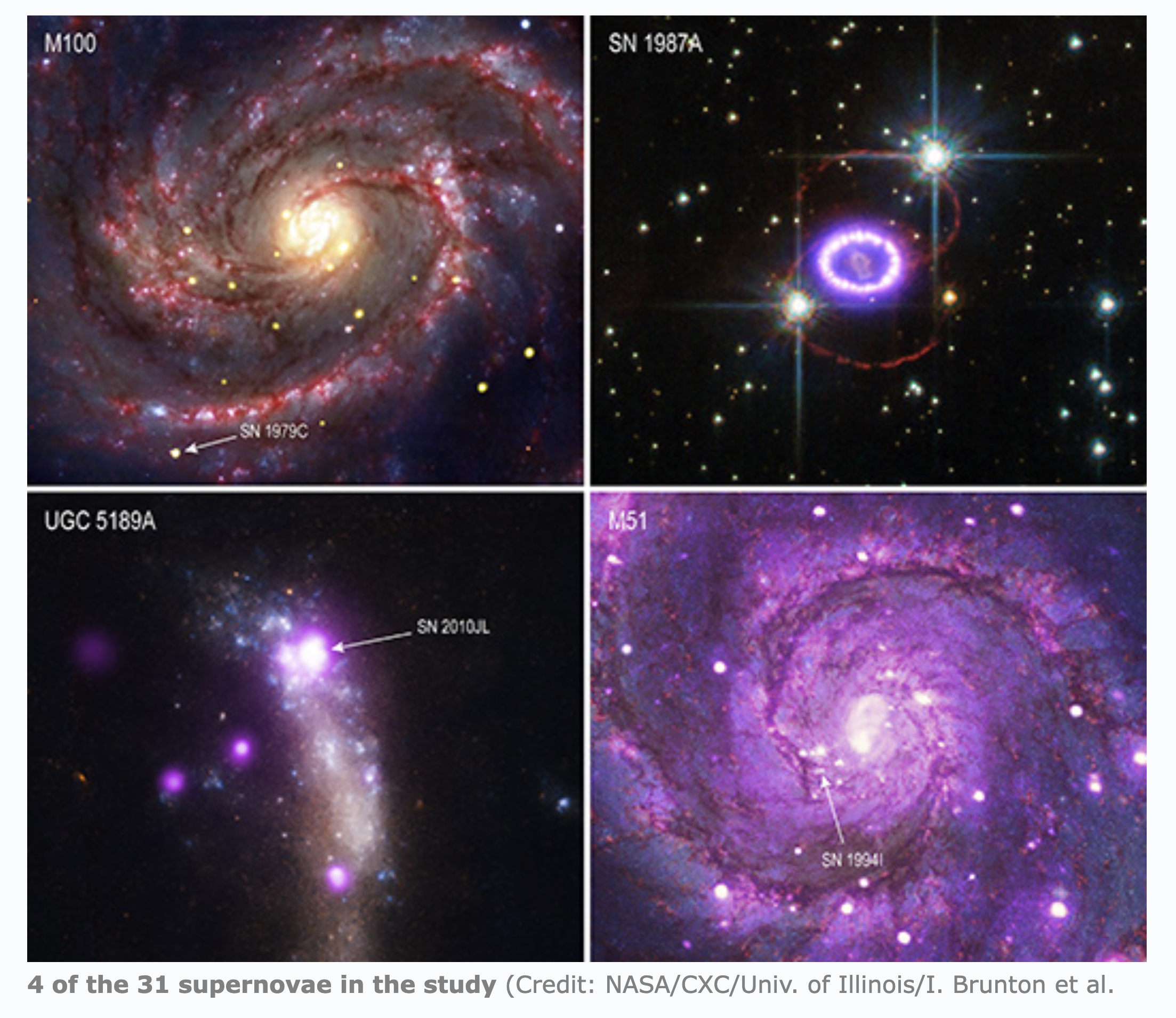
SN 1987A (click for a CHANDRA Youtube video)--
SN 1987A is a Type IIp SN that exploded
in the Large Magellanic Cloud roughly 166,000 years ago but was
detected on Earth only 37 years ago, on 23 February 1987. SN1987A, the last
historical supernova was an atypical supernova in that its progenitor star
was a blue supergiant, Sanduleak SK -69 202, not a red supergiant. Consequently,
SN 1987A was fainter than a typical Type II supernova.
SN 1987A is the only historical supernova to have taken
place in the modern technological era.
SN 1987A has been studied over the electromagnetic spectrum from the
γ-ray to the radio and was detected in neutrinos.
|
Recall that the energy budget (the total energy released in a Type II Supernova) is
about 3x1046 Joules, more than 100 times the amount of energy the Sun releases over
its entire 10 billion year lifetime,
1.3x1044 Joules.
- Interestingly, 99% of the energy comes out as neutrinos posing no threat
to us, while way less than 1 %
- about 1 % of the energy is the kinetic energy carried by the ejected
material, the blast wave. Supernova may eject many solar masses of material
traveling at very high speeds, 5,000 to
10,000 km per second. These ejecta drive shock waves that carry energy and
cause production of copious amounts of x-rays
when they collide with surrounding material at later times.
See the pictures of SN 1987A
and other young supernovas
- less than 0.01 % of the energy comes out as the prompt γ-rays,
ultraviolet,
visible, and other forms of high-energy electromagnetic radiation that
bursts into the view when the shock wave erupts out of the star
- a small amount, but perhaps percents of the energy may
come out as high energy particles, cosmic rays;
cosmic rays may form in the shock waves produced
as the gas ejected from the
exploding star rushes through the surrounding medium. Most of these
particles have energies less than a million-th of a Joule, but the most
energetic ones can have energies of 100 Joules!
The questions that need to be answered are then
How do the different forms of energy from
supernovas affect us on Earth?
(Nice
Nice PBS video on Supernovas and Earth)
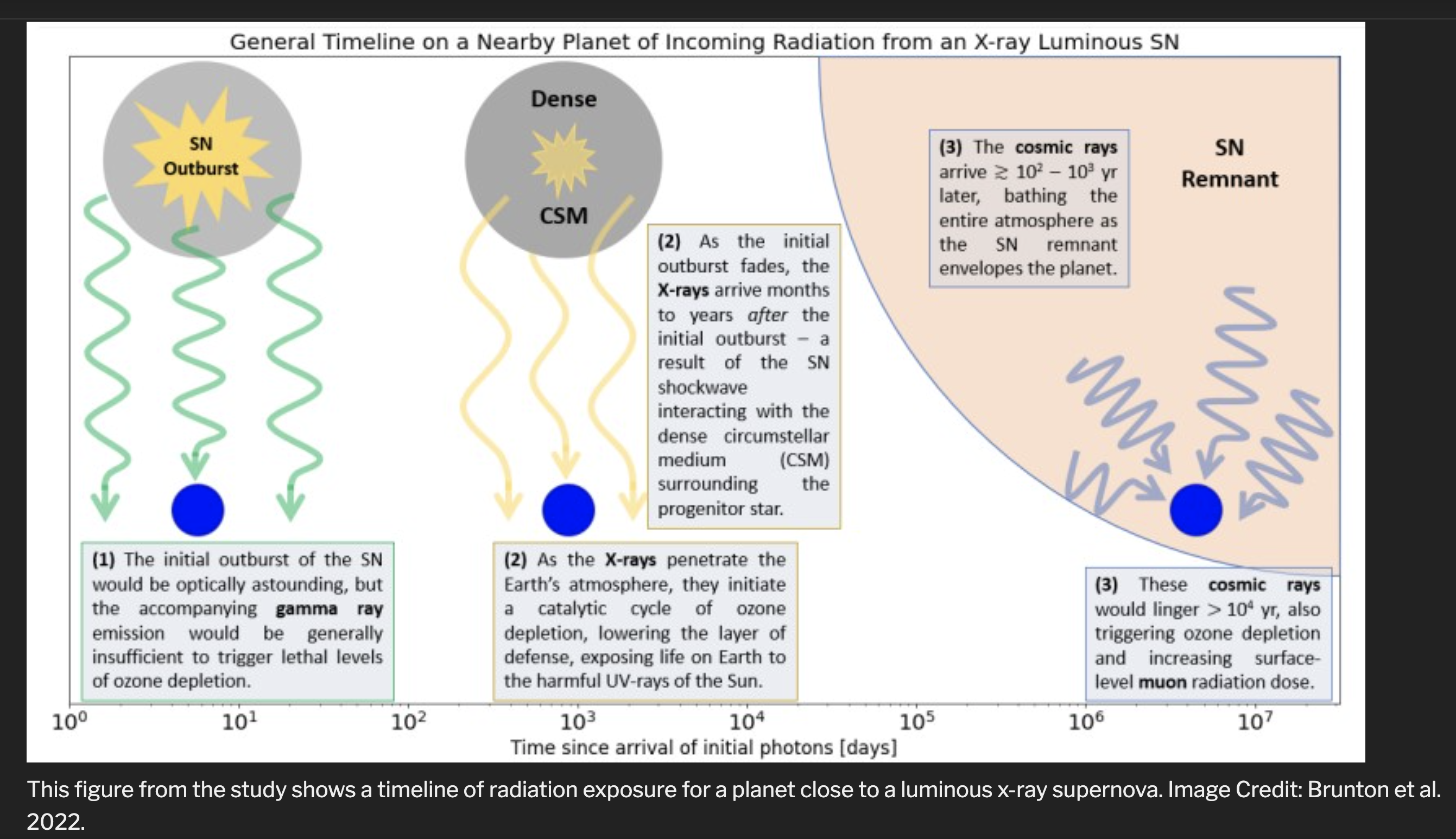
1. Initial Burst of X-rays and γ-rays
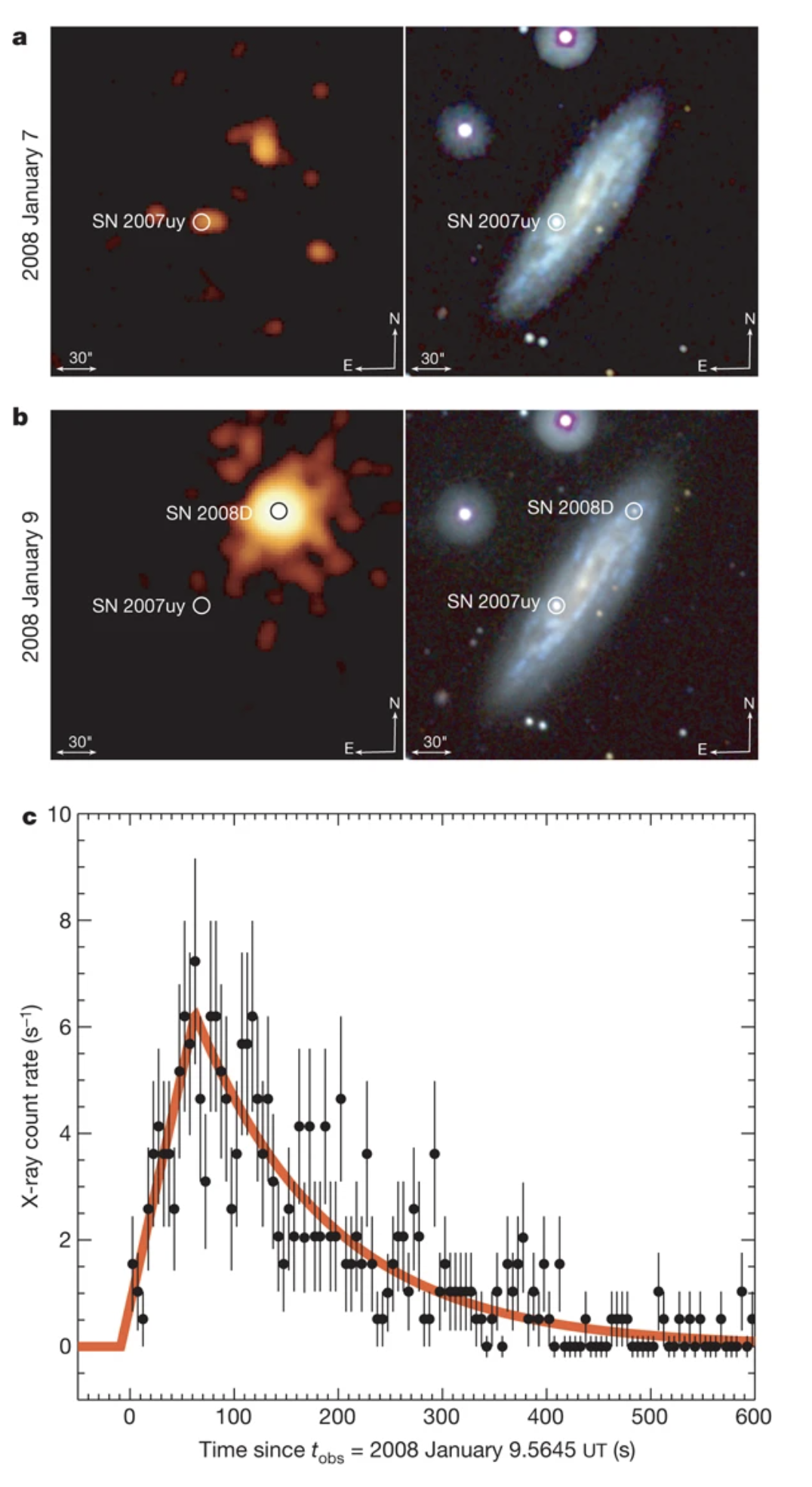 |
An extremely luminous
x-ray outburst took place at the birth of
Supernova SN 2008D, 9 January 2008. The x-ray burst arising from shock
break-out was huge, 6x1036 W! Recall that the luminosity of the
Sun over all energies is 4x1026 W.
Was a monster event this large, large enough
to do us damage to us? Well let's think about it for a minute. Consider
a big critter like a dinosaur. Would a supernova this large affect such a
critter? Well, let's see.
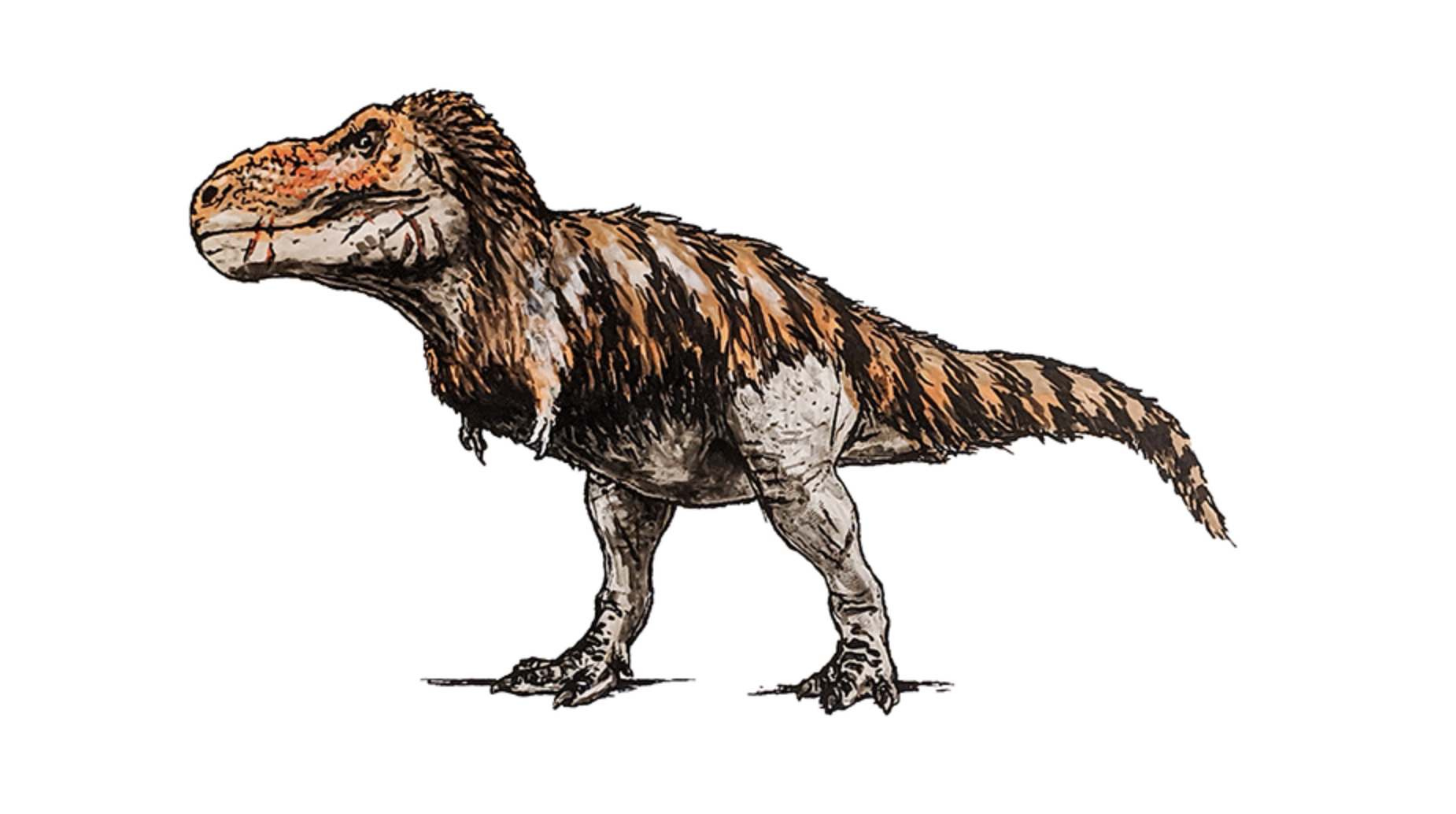
How much x-rays would this T Rex absorb?
- The flux of x-rays at Earth is
F = L/(4πd2) = 300 W per square meter, if the supernova was
at a distance of 4.3 light years, the distance to Proxima Centauri the star
closest to us outside of the Sun. A
T Rex is typically about 12 feet high and
40 feet long and so, we estimate that it has an absorbing surface area
of about 85 square meters and so absorbs ~27,000 W.
- The other important
property is how much does the T Rex weigh, because this energy is spread
over all of the cells in the T Rex. A typical T Rex weighs
about 6,000 kilograms and so the T Rex absorbs around 4.5 Joules
per kg every second.
Is this amount
of x-rays that is dangerous?
Well, let's define the unit that measures how much radiation an object
absorbs. We set 1 rem = 10-2J absorbed per kg. In terms of
a rem, the T Rex absorbs 450 rem every second for a supernova that went off
4.3 light years from the Earth. This is a lot of radiation. The T Rex would
absorb more than a lethal dose of radiation every 1.3 seconds
(a lethal dose of radiation is around 600 rem).
Only a dose of 5 rem per year is considered safe.
If the shock breakout lasts for several
hours, then a T Rex would only receive a lethal dose of radiation for
supernovas that are within a few tens of light years from the Earth!
The γ-ray outburst associated with the x-rays may also affect the
ozone layer of the Earth.
The high-energy γs can break apart N2 molecules in the
Earth's stratosphere
leading to the formation of molecules composed of N and O, for
example, NO. The NO molecules act as catalysts for reactions that can destroy
ozone, O3, and so destroy the ozone layer of the Earth.
The prompt burst of γ-rays is again not large
enough to cause depletion of the ozone layer of the Earth
for supernova farther than tens of light years away.
|
2. X-rays From Blast's Interaction with Surrounding Disk or Material
 |
Aside from the prompt x-rays produced in the explosion, another burst of x-rays
may come when the ejected material collides with surrounding matter.
The strong shock generated in the collision
superheats the material to high temperatures leading to emission of x-rays.
These x-rays may also affect the ozone layer.
The high-energy x-rays
can break apart the N2 in the stratosphere
of our atmosphere leading to the formation of molecules composed of N and O,
NO. The NO molecules again act as catalysts for the destruction of ozone,
O3.
Are these x-rays strong
enough to cause depletion of the Earth's ozone layer?
The recent study by NASA's CHANDRA x-ray observatory of 31 Supernova's has shown
that supernovas are dangerous to our ozone layer out to distances of 130 light
years or so (farther than the for the prompt x-rays,
but Betelgeuse still sits well outside this limit).
|
3. Cosmic Rays
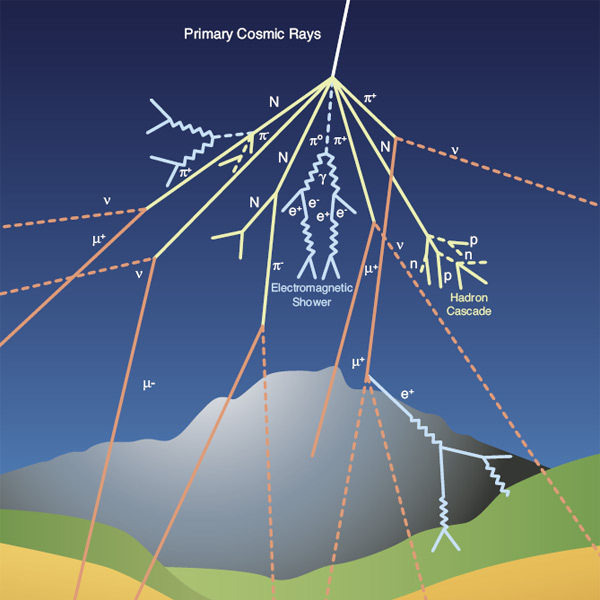 |
Aside from the γ-rays and x-rays produced by supernova explosions,
high energy particles, cosmic rays are produced
by supernova when the shocks produced during their supernova remnanat phases
collide with surrounding matter.
The collisons can acclerate particles, mainly
protons (hydrogen nuclei) to nearly the speed of light. These high energy
particles are called cosmic rays.
- Some cosmic rays can
have energies larger than 15 Joules! This is a huge amount of
energy; a single particle that carries the weight of a falling shoe. To
understand how large this is, note that a falling shoe contains
more than 1027 particles! Each particle in a falling shoe carries
1.5x10-26 Joules!
- The bulk of the cosmic rays
have much smaller energies, typically less than
one million-th of a Joule.
Cosmic rays may also
affect the ozone layer of the Earth. At high altitudes in our
atmosphere, cosmic rays may collide
with nitrogen molecules in the process breaking them apart and
again leading to the production of NO molecules. The NO molecules may
then again catalyze reactions that destroy our ozone layer.
Cosmic rays
can deplete the ozone layer of the Earth for
supernovas out to distances of 30-50 light
years or so.
Recent work further suggests that
cosmic rays may also cause
trouble when they produce the particles known as muons,
μ, near the surface of the Earth. It has
been suggested that μs could penetrate into the upper levels of our
oceans and produce mini-mass extinctions in large marine critters.
|
4. Was There a Supernova within 150 Light
Years from the Earth 2.6 Million Years Ago?
 |
This is an interesting question because 2.6 million years ago
marks the end of the Pliocence era which saw changes in the climate of the Earth. Recent work has suggested
a supernova may, in fact, occurred 150 light years from Earth and triggered climate change
and the mass extinction of large marine mammals.
How could we go about showing that a supernova took place near the Earth 2.6 million years ago?
Is there evidence a supernova took place near the Earth around 2.6 million years ago?
|

| |
We can determine whether or not a supernova happened nearby through examination of the fossil record
on the Earth. A quite useful way is to study layers of sedimentary found in ocean basins. An example
of such sedimentary rock is the formation found in Utah.
One can see that sedimentary rocks are laid down in
layers with the youngest layers lying to the top. Ocean basins are young in a geological sense, but still
have ages up to 100s of millions of years and so easily hold the record of events that take place within
5 million years.
So, what can we do?
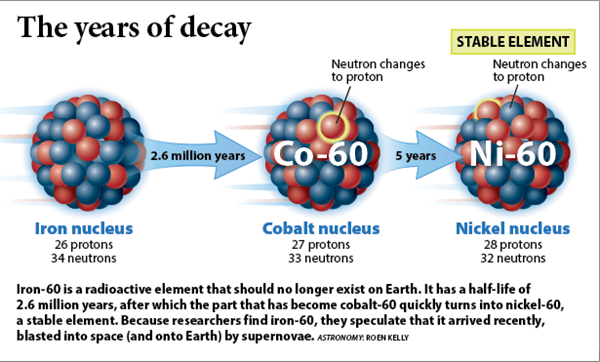
In supernova outbursts radioactive elements are produced, we have already met nickel 56 and how it
decays to produce SN lightcurves. There are other kinds of radioactive elements produced. For the problem at hand,
a prime example is iron 60 which decays to cobalt 60 and then to nickel 60
(see left) with a half-life of 2.6 million years.
|
The strategy is clear. Because the Solar System (and Earth)
is 4.5 billion years old, any iron 60 that was around
when the Solar System was born will have long ago decayed to
nickel 60. Therefore if there is any iron 60 left, we
know that it must been incorporated into the Earth within the
last millions of years, the exact amount of time
depending upon how much of it has changed into nickel 60.
- Suppose a rock is born with 64 atoms of iron 60
- After one-half life, 2.6 million years,
one-half of the iron 60 will have changed to nickel 60. That is, we
will now have 32 atoms of iron 60 and 30 atoms of
nickel 60. Judging from the relative amounts of iron 60 and
nickel 60 will allow us to infer the number of
half-lives that elapsed and so the age of the rock.
- After another half-life, one-half of the
remaining iron 60 will decay to nickel 60. That is, 16 of the
32 remaining iron change to nickel 60. After two half-lives,
there will then be 16 iron 60 atoms and 32+16
nickel atoms.
- We can use this method to determine the age of a
rock through comparison of the amount of nickel 60 it
contains to the amount of iron 60 it contains.
This method is known as Radioactive
Age Dating.
Recent work has suggested that perhaps two supernovas took place
near the Earth, one 2.6 million
years ago and one 7 to 8 million years ago.







