 Pieter Bruegel the Elder (1525-1569) |
|
 Pieter Bruegel the Elder (1525-1569) |
|
 |
|
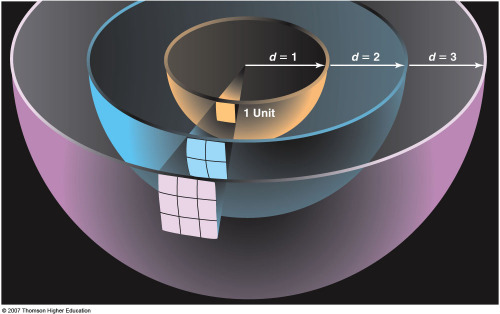 |
There is more to the story than this, however. Because of the large distance to the Sun (150,000,000 km), we intercept only ~2.2x10-5 of the Sun's power per unit area (its energy Flux). The brightness of the Sun (its flux) falls off as 1/D2, where D is the distance to the Sun. Although the amount of energy we intercept because of this effect (the inverse square fall-off of the brightness of the Sun) is tiny, the fraction of the Solar power we absorb is large in the sense that the Earth easily intercepts enough energy from the Sun to satisfy our energy needs. Even allowing for cloud cover (Albedo effects) and the absorption of light in our atmosphere (our atmosphere is not transparent because of opacity effects), the energy which reaches the ground is substantial, ~0.34 kWatts per square meter, A Solar collector ~100 miles x 100 miles in size is capable of capturing enough Solar energy to satisfy the current energy needs of the Earth. |
 |
|
 |
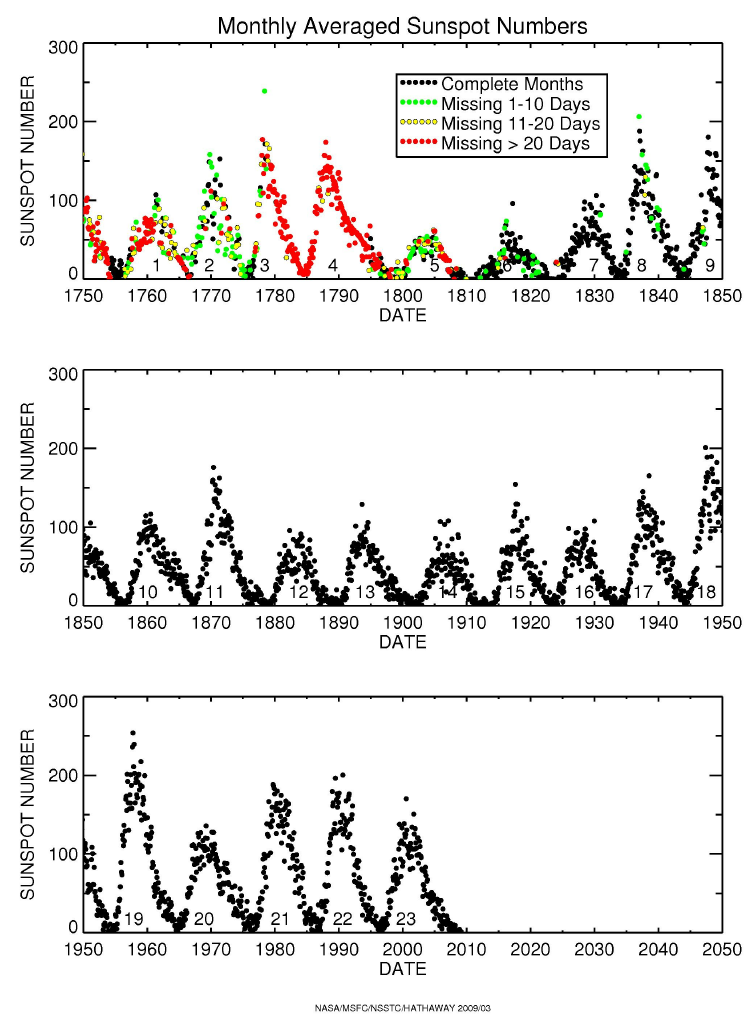 |
|
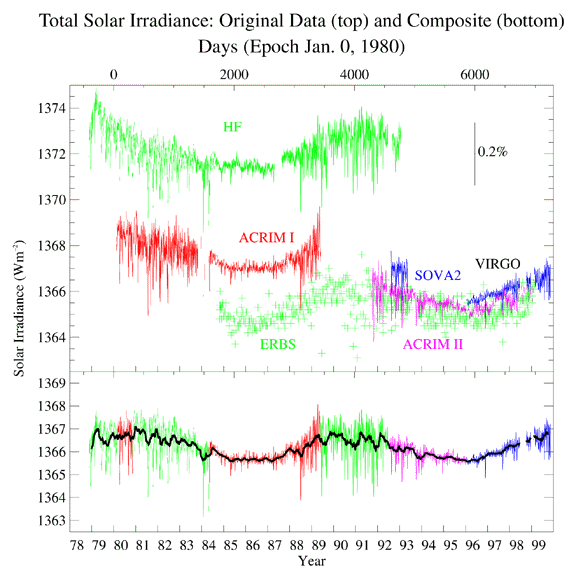 |
|

|
|
The answer to the question of then, why do we have liquid oceans? requires that our atmosphere in the past had a much different chemical composition than today so that the Greenhouse Effect could maintain liquid oceans or, perhaps, the Sun was much brighter in the past than we now believe.
 |
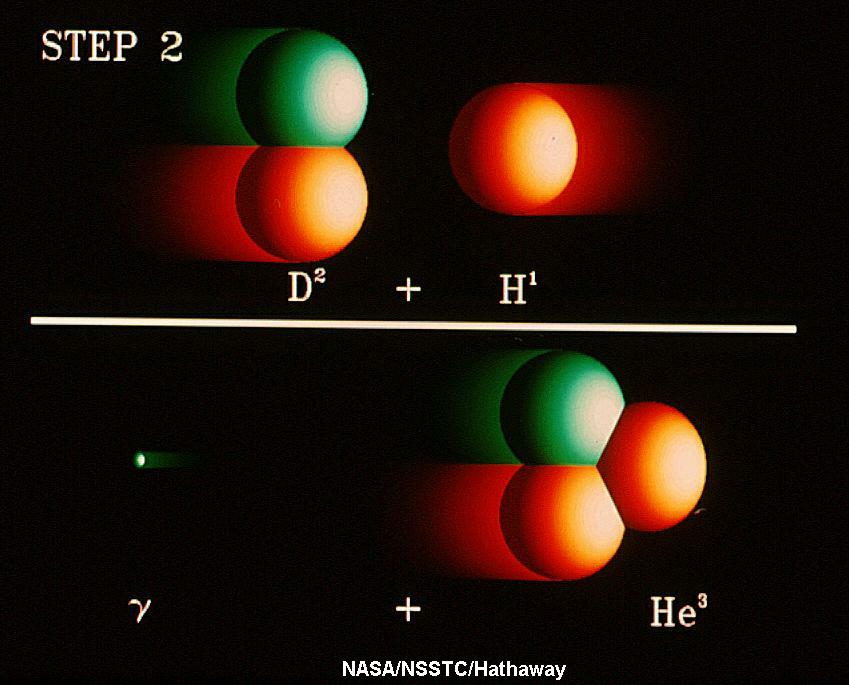 |
 |
 |
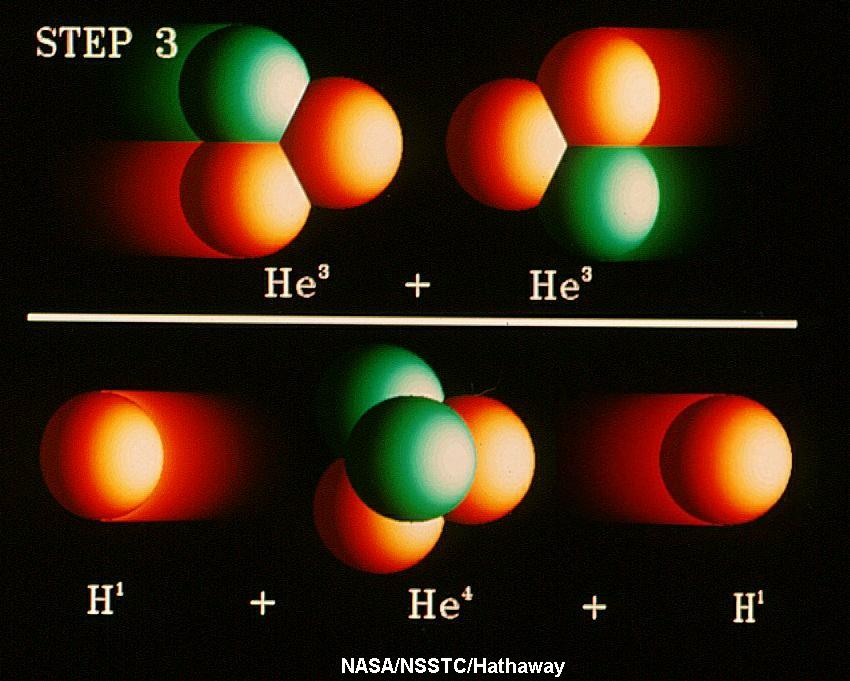
Solar Neutrino experiments were started in the 1960s by Brookhaven scientist, Ray Davis to verify that we understood how the Sun worked. No one thought that the experiment would that interesting; it would be difficult but the result would not be surprising. It came as a rude surprise when Davis's experiment detected fewer neutrinos than predicted by the best models of the Sun, throwing doubt onto whether we really did understand our Sun. Follow-up experiments also found ~1/3-1/2 of predicted neutrinos. This conundrum persisted for ~35 years until the early 2000s when, first, the Super-K (Super Kamiokande) experiment showed neutrinos were chamaeleon-like in nature. Neutrinos, once produced, could change into forms undetectable by the early experiments. The SNO (Sudbury Neutrino Observatory) experiment, able to detect transmuted neutrinos, then came online and detected the predicted number of Solar neutrinos. The amusing result was that a simple observation of the Sun led us to a deeper understanding of how the Universe works on the sub-nuclear scale! |
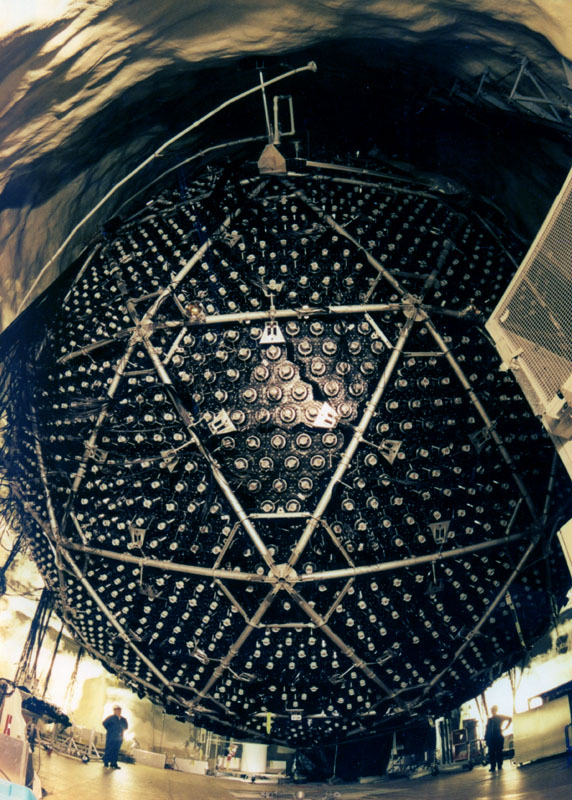 |
Comment: The Davis experiment was a remarkably difficult experiment. The vat contained ~400,000 liters of cleaning fluid (tetrachloroethylene). This vat contains ~2x1030 chlorine atoms. The neutrinos interact with the chlorine (on rare occasions) transforming the chlorine to radioactive argon which is subsequently detected. A huge number of neutrinos passes through the vat every second, 400 billion neutrinos flow through the detector per square inch per second. Remarkably, one expects to build up only a few tens of Argon atoms every month! This exceedingly difficult experiment was performed accurately enough by Davis to show that there were roughly 1/3 the number of neutrinos passing through his experiment as was predicted. Davis received the Nobel Prize (along with Dr. Koshiba of the Super-K experiment) in Physics in 2002 for this remarkable work.

|
|