Atomic Physics
 |
Atomic Physics
|
Based on the experiments of Bunsen and
Kirchhoff, it was
known that the wavelengths (energies) of the spectral
lines for particular
elements were the same every time they were measured
and the patterns of the lines were unique to one element.
The lines
thus serve as fingerprints for atoms.
The element to which a given atom corresponds is defined by the number
of protons it contains in its nucleus. The number of electrons and neutrons
is irrelevant in terms of the determination of the type of chemical element.
The number of protons in an atom (nucleus) is given by the Z of the
nucleus. We have
Element | Number of Protons in Nucleus, Z |
hydrogen |
1 |
helium |
2 |
lithium |
3 |
berylium |
4 |
| | and so on |
For the rest of the periodic table, see
here.
So, how do the neutrons fit into this scheme?
Whether an atom contains zero neutrons or many neutrons in its nucleus does not
affect the type of element it is; it is the number of protons which determines
the chemical element. The neutrons
do affect the mass of the atom (its atomic weight, A).
To set the jargon, consider hydrogen with Z = 1 (1 proton).
- A hydrogen can have 0 neutrons, and its weight is A = Z + # of
neutrons = Z = 1
- A hydrogen can have 1 neutron, and its weight is then A = 2 (and is called
deuterium).
- A hydrogen can have 2 neutrons, and its weight is then A = 3 (and is called
tritium). Tritium
is highly radioactive (unstable).
All of the above are isotopes of hydrogen.
The mass of a proton is ~1,836 times that of an electron while neutrons
and protons have roughly the same mass ===> the
mass of an atom is contained in its nucleus while the nucleus is only
(1 fermi)3/(1 A)3 ===> 10-15
of the volume of the atom.
Atoms are mostly empty space.
If we make an analogy with the Solar System,
we can imagine that the nucleus is the Sun, and the electrons
are the planets. The electrical force plays the role of gravity.
This analogy is useful, however, there are important differences between how
our Solar System works
and how an atom works.
- In our Solar System, the planets can, in principle,
move about the Sun in orbits of any size.
- In an atom, the electrons
cannot orbit about the
the nucleus in any old orbit.
There is a set of
well-defined orbits in which electrons can move
===>
electrons can exist only with well-defined amounts of energy in an atom.
The above properties of atoms are not intuitive and it wasn't until the
twentieth
century when they were reasonably well-understood and could be modeled.
The physics used to model these and other related
phenomena is referred to as Quantum Mechanics. We will have
more to say about Quantum Mechanics in the future. For now consider
the following representation for the structure of an atom:
We will use the well-type picture to represent
an atom.
Hydrogen
The hydrogen atom is particularly
attractive to theorists because
of its simplicity. Its most common
form (isotope)
contains one proton and one electron. Here are some facts
about hydrogen atoms
- The energy needed to strip an electron from the lowest level
is 2.2x10-18 Joules. This is a tiny number and so a
different unit is used
the electron volt.
There are around
6x1018 electron volts in one Joule!!
In terms of electron volts, the energy
required to remove an electron from the bottom level of a
hydorgen atom is 13.6 electron volts.
(The process of removing an electron from an atom is known as
ionization.)
- The energy needed to strip electrons from higher levels in the
well is easily found. Bohr showed that the energy needed was given by
where n is the level. The lowest level is n
= 1, the next higher
level is n = 2, and so on.
- electrons exist only in
certain levels (energy states), an atom cannot absorb photons
with arbitrary energies.
Due to conservation of energy, an atom can
only absorb an amount of energy which just promotes an electron to a higher
level. (What does this say about the production of absorption and emission
lines?) The energy of a photon capable of inducing a transition
between levels
n = 1 and n = 2
is
Energy = 13.6 eV/11- 13.6 eV/22 = 10.2 eV
Because the strength of the electrical
force depends upon the charge of the
nucleus (number of protons) and the number of electrons,
different atoms
have different energy structures.
Absorption and Emission of Photons

Note that the largest
transitions (longest arrows) require the highest energy
photons, particles of light, because the
transitions have the largest changes in energy.
The photons which produce the
largest transitions therefore involve
the photons with the shortest wavelengths (since E = hc/W = hf).
- If an electron jumps to a higher energy level, it must absorb
energy and
this corresponds to an absorption line
- If an electron drops to a lower energy level, it must get rid of
energy and this corresponds to the production of a photon (an emission
line is produced).
- If the electron leaves the atom, the atom is ionized.
Comment: (i) A neutral atom is denoted by I. An atom which has lost 1 electron is
denoted by II. An atom which has lost 2 electrons is denoted by III, and so on.
For example, neutral hydrogen is HI; singly ionized hydrogen is HII.
Comment: (ii) To ionize an atom, all we
require is that the photon have enough energy to lift the electron
out of the well. If the photon has more than this threshold energy, it
simply gives the excess energy to the electron (as kinetic energy).
So, in terms of the appearance of the spectrum, we find that there is a
threshold where ionization begins followed by a broad trough which extends
to shorter wavelengths
(higher energies).
Such
ionization edges
are seen in the spectra of many stars.
- If an electron is captured by an atom, we say that a
recombination has taken place
Some definitions:
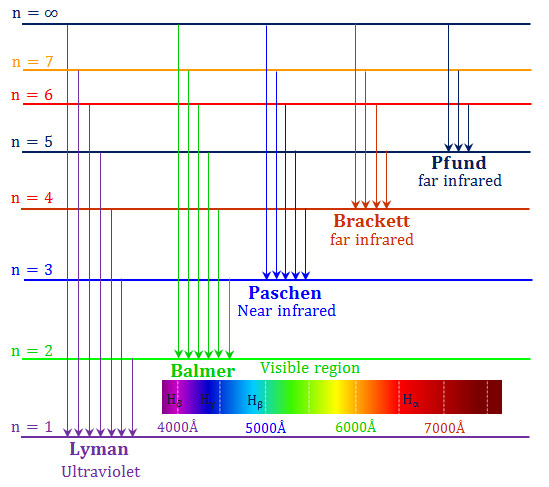
- Lyman line:
An electron starts from n = 1 and jumps to a higher level or
an electron drops from a higher level to n = 1,
- Balmer line:
An electron starts from n = 2 and jumps to a higher level or
an electron drops from a higher level to n = 2,
- Paschen line:
An electron starts from n = 3 and jumps to a higher level or
an electron drops from a higher level to n = 3,
- Brackett line:
An electron starts from n = 4 and jumps to a higher level or
an electron drops from a higher level to n = 4,
- Pfund line:
An electron starts from n = 5 and jumps to a higher level or
an electron drops from a higher level to n = 5,
Lyman lines fall in the ultraviolet.
Balmer lines fall in the optical.
Paschen, Brackett, and Pfund lines fall in the
infrared.



