Astronomy 122
Mid-term Exam I Review
January 13, 2015, jeb
Exam will cover
Fundamentals
-
Scientific Notation
Know it.
- Scientific prefixes
- kilo (1000) like kilometer = 1000 meters
- Mega (106) like Megaparsec = 1,000,000 parsec
- milli (10-3) like millimeter = 0.001 meters
- micro (10-6) like micrometer = 0.000001 meters
- nano (10-9) like nanometer = 10-9 meters
- Distance Scale
- Light-year (9.46 x 1012 kilometers, or about 1013 kilometers)
- Parsec = 3.26 Light-years
-
Temperature Scales
-
Some Equations
The Stars and our Sun
The elements created in the Big Bang were mostly hydrogen and helium
- The Big Bang occurred about 14 billion (13.8 x 109) years ago
Stars are born within dark clouds in interstellar space
The Sun's source of energy is thermonuclear fusion, the fusion of
atomic nuclei with the associated release of energy
- This process of fusion creates the heavy elements (such as carbon, nitrogen, and oxygen)
from the elements hydrogen and helium
The Sun is approximately 5 billion (5 x 109) years old, and it will continue generating
energy much as it is today for another 5 billion years
Nearby Stars
- Closest - Alpha Centauri System
- Three stars
- 4 light-years away
Brightest in Earth's sky - Sirius
Stellar deaths may be violent, such as the supernova explosion observed in the Large Magellanic Cloud
in 1987 (SN1987A)
The "star dust" that results from such explosions is the source of heavy elements, making possible the
existence of all things, planets, moons, animals, and plants.
Light, Electromagnetic Radiation, and
Spectroscopy
- Wavelength
-
Spectrum (long wavelength -> short)
............(low energy -> high energy)
- Radio(greater than 100 microns- or 100 x 10-6meters)
- Infrared(between 100 microns and 0.7 micron)
- Visible(between about 0.7 microns and 0.4 microns)
. . . (or between about 700 nanometers(nm) and about 400 nm)
- Ultraviolet(between about 400 nm and about 10 nm)
- X-ray(between about 10 nm and about 0.01 nm)
- Gamma-ray(below 0.01 nm)
- All electromagnetic radiation travels at the speed of light
. . . . . . . (300,000 kilometers/second)
- Radiation is composed of "photons" (packets of electromagnetic radiation)
- the "photons" behave as particles sometimes, and as waves at other times
- the "photons" have energies related to the "color" or wavelength
- short wavelengths -> higher energies
- long wavelengths -> lower energies
where E is energy (in Joules)
h is Planck's constant
(h = 6.63 x 10 -34Joule seconds)
and f is frequency in 1/sec, or Hz.
Photon energies are very small
For example, for visible light (0.5 μ m), f = 6 x 1014 /sec
|
wavelength x frequency = velocity = 300,000 km/sec |
|---|
So, E = 4 x 10-19 Joules
- "Windows" in the atmosphere
-
Generation of Electromagnetic Radiation
Electromagnetic Radiation is generated by the movement of charged particles
- Electrons (negative charge) are most important
-
Blackbody Radiation
-
Peak wavelength decreases with temperature (Wien's Law)
- Total energy output increases with temperature
...........(increases as T4 - Stefan's Law)
- Surface temperature of the Sun is 6000 K
...........--> peak of spectrum in the visible light
-
Inverse Square Law
The apparent brightness of a star is inversely proportional to the
square
of its distance.
- Doppler Effect
- Wavelength of light from moving object is shifted
- Moving away ->
Red shift
- Moving toward ->
Blue shift
-
Absorption and Emission Lines
-
Kirchhoff's Laws
- Continuous spectrum
- Absorption-line spectrum
- Emission-line spectrum
-
Atoms and Molecules
- Photons
- The particle nature of electromagnetic radiation
- represents wave-particle duality
- photons can
be absorbed
or emitted by an atom,
boosting the electron to an excited
state (on absorption)
or bringing the electron to a lower energy state (on
emission)
- Since only certain energy states of the atom are allowed, only certain
wavelengths of photons are emitted or absorbed, explaining the spectral
lines.
-
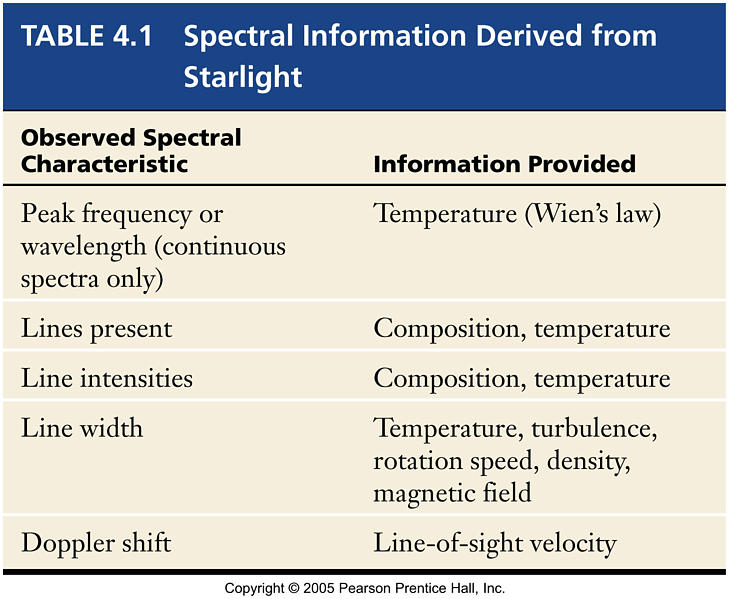
Spectral Analysis
- Basic Optics
-
Refraction
- The bending of light as it crosses the boundary from one transparent material to another
- Red (long wavelength) bends less than blue (short wavelength)
Line Broadening
- Line broadening is caused by the environment in which the emission or absorption occurs