Absorption and Emission Lines
|
The electromagnetic radiation received from an object in space
is composed of many different wavelengths
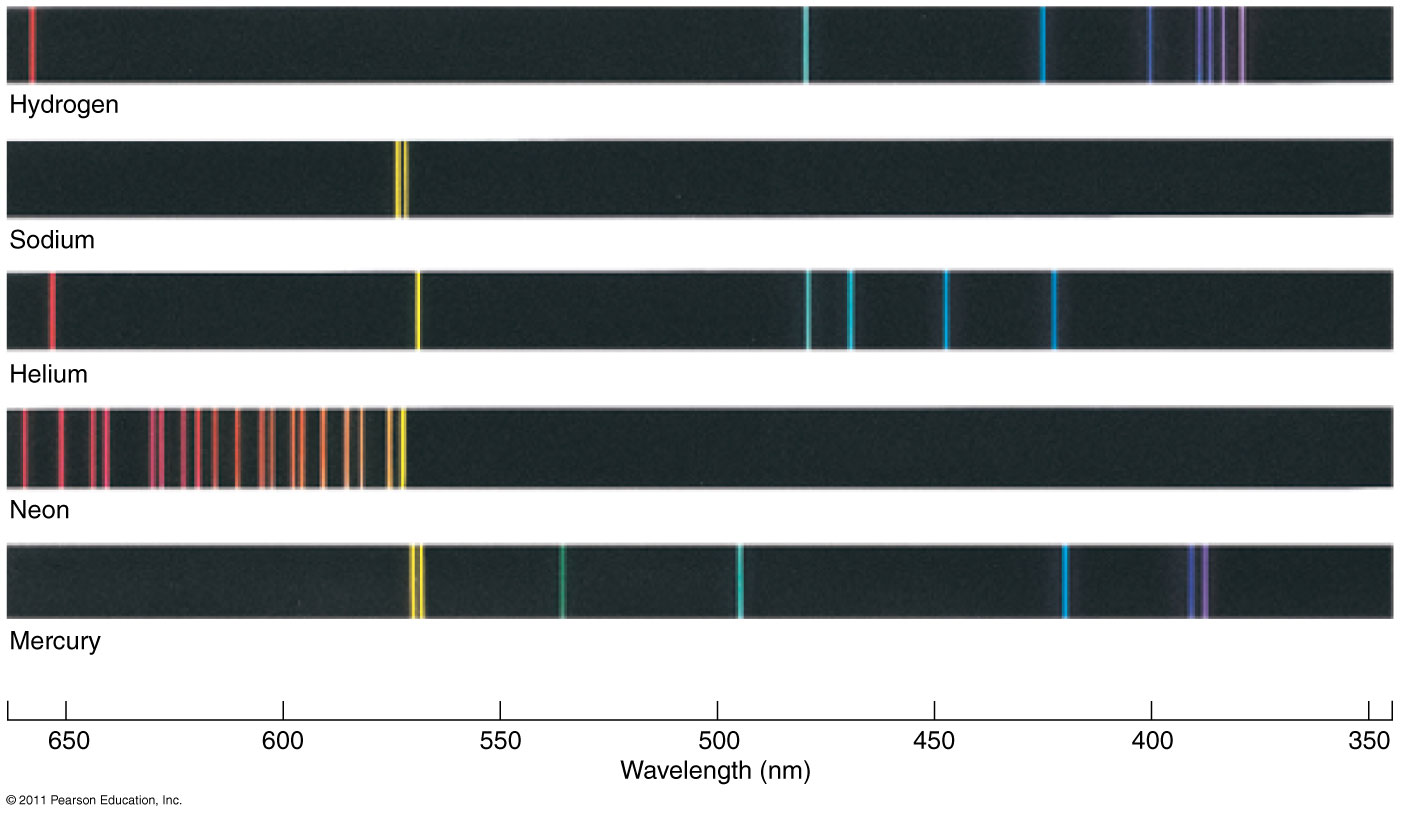
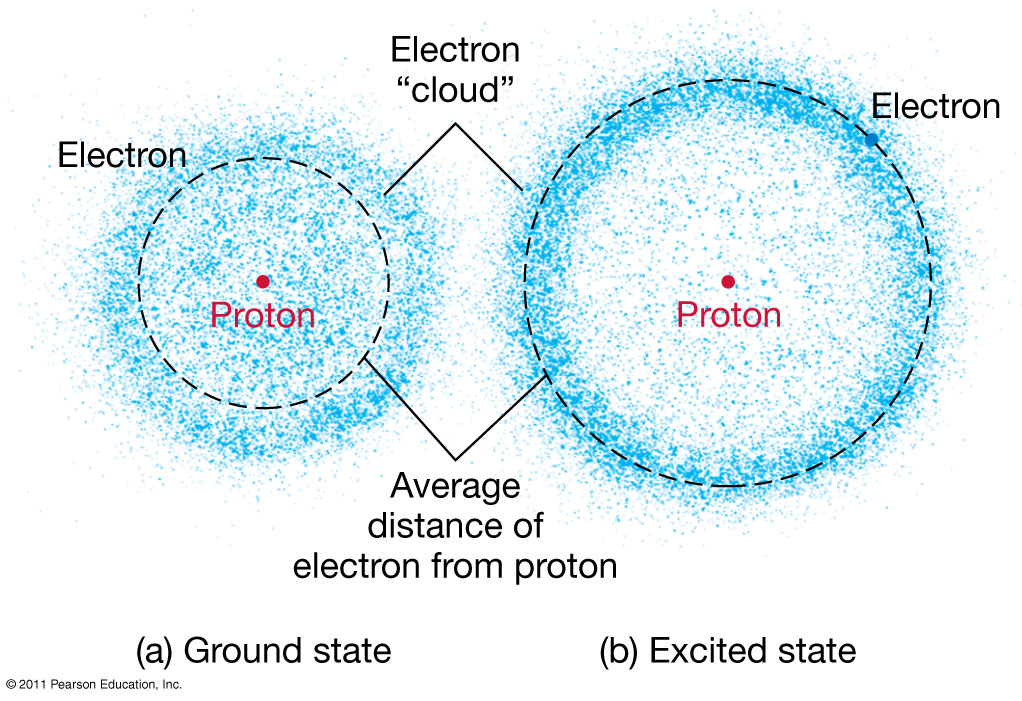
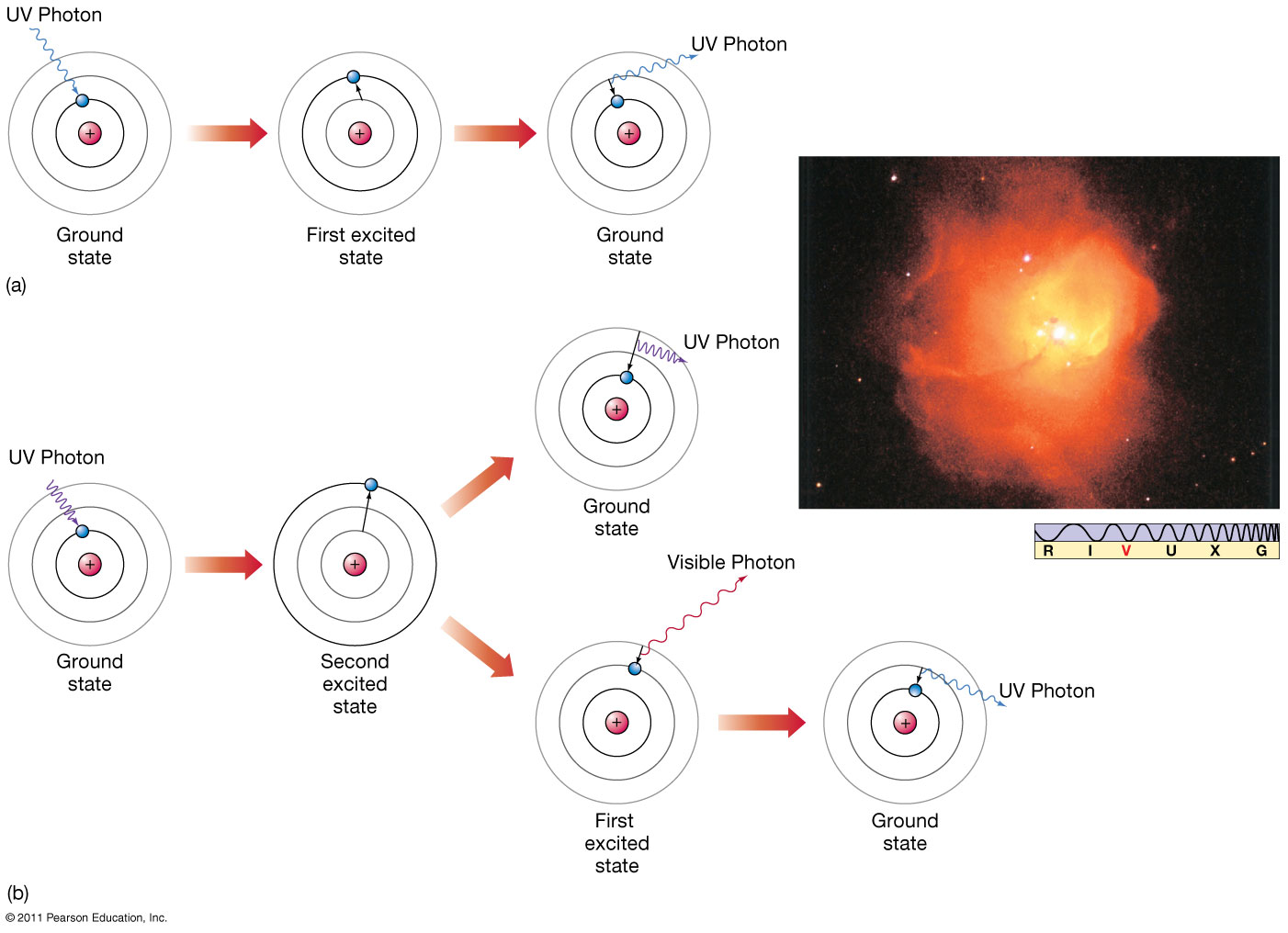
Splitting the incoming radiation into its component wavelengths is a vital step in the process of analyzing the radiation to obtain information about the distant object A spectroscope is a device for splitting a beam of radiation into its component frequencies and delivering them to a screen or detector for detailed study Simple spectroscope Source of continuous radiation gives rainbow, while hydrogen gas emits spectral lines Emission spectra of some well-known elements. Illustration of formation of absorption lines Comparison of the absorption and emission lines of sodium Dark absorption lines of the Sun | 
|