|
Ideal Gas Law:
|
Readings:
Schneider & Arny: Unit 49
|
|
(Audio Lecture)
|
Stars are basically large balls of hot hydrogen gas, and the macroscopic
properties of a hot gas are governed by the Ideal Gas Law of
chemistry.
An ideal gas is a gas that conforms, in physical behavior,
to a particular, idealized relation between pressure, volume, and
temperature. The ideal gas law is a generalization containing both
Boyle's law and Charles's law as special cases and states that for a
specified quantity of gas, the product of the volume, V, and pressure, P,
is proportional to the absolute temperature T; i.e., in equation form, PV
= nkT, in which k is Boltzmann's constant and n is the density of the gas.
Such a relation for a substance is called its equation of state and is
sufficient to describe its gross behavior.
Although no gas is perfectly described by the above laws, the behavior of real
gases is described quite closely by the ideal gas law at sufficiently high
temperatures and low pressures (such as air pressure at sea level), when
relatively large distances between molecules and their high speeds overcome any
interaction. A gas does not obey the equation when conditions are such that
the gas, or any of the component gases in a mixture, is near its triple
point.
The ideal gas law can be derived from the kinetic theory of gases and relies on
the assumptions that (1) the gas consists of a large number of molecules, which
are in random motion and obey Newton's deterministic laws of motion; (2) the
volume of the molecules is negligibly small compared to the volume occupied by
the gas; and (3) no forces act on the molecules except during elastic
collisions of negligible duration.
While all the above conditions are not strictly true, (where they breakdown
interesting things happen - such as friction) in general the behavior of matter
is well described by this kinetic theory. Temperature is explained by atomic
theory as the motion of the atoms (faster = hotter). Pressure is explained as
the momentum transfer of those moving atoms on the walls of the container
(faster atoms = higher temperature = more momentum/hits = higher pressure).
Almost all the behavior of normal stars is given by the simple relations of
the ideal gas law. For example, as a star shrinks, the volume decreases
and the pressure increases (this is backwards from a balloon cause the
pressure in a balloon works from the inside out, pressure in a star is due
to the weight of the outer layers pushing down).
With four variables, it becomes very hard to predict how pressure, volume,
temperature and density will behave if you change just one. Usually, we
hold one of them constant (like getting the temperature the same) and
explore the behavior of the other three. Some relations are fixed by the
laws of matter, for example if you hold the volume constant (like a steel
ball) then the density must remain constant cause the mass does not change
(nothing gets in or out) and the volume does not change. If the equation
is confusing, the following summarizes the way the math works:
- Holding volume constant, as pressure goes up --> temperature goes up
- Holding volume constant, as pressure goes down --> temperature goes down
- Holding pressure constant, as volume goes up --> temperature goes down, density goes down
- Holding pressure constant, as volume goes down --> temperature goes up, density goes up
- As temperature goes up --> pressure goes up, volume goes up, density goes down
- As temperature goes down --> pressure goes down, volume goes down, density goes up
It helps to think about balloons and what happens when you heat or squeeze
a balloon (squeezing lowers volume, increases pressure).
Stellar Structure:
Stars form from clouds of gas and collapse under self-gravity. The
collapse is stopped by internal pressure in the core of the star.
During the collapse, the potential energy of infalling hydrogen atoms
is converted to kinetic energy, heating the core. As the temperature
goes up, the pressure goes up to stop the collapse.
The heat from the collapse is sufficient for a star to shine, but
only for a timescale of 15 million years (called the Kelvin-Helmholtz
time). Since most stars are over 10 billion years old, then they
must be producing its own energy rather than shining on leftover
energy from formation.
The structure of stars is determined by 5 relations or physical
concepts:
- hydrostatic equilibrium - Most stars, like the Sun, are not
expanding nor contracting. They are stable in size. Therefore, this
fact means that the internal pressure must balance the weight of the
material above it (self-gravity)
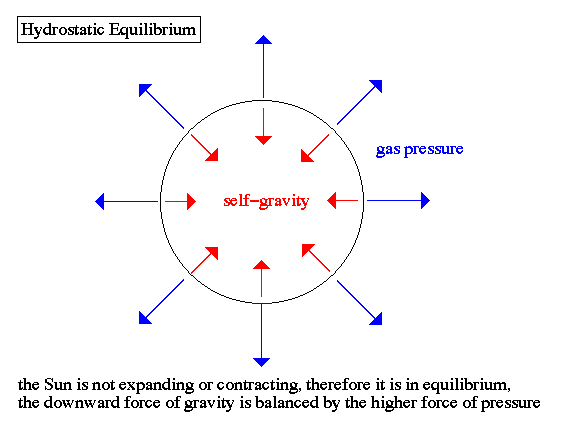
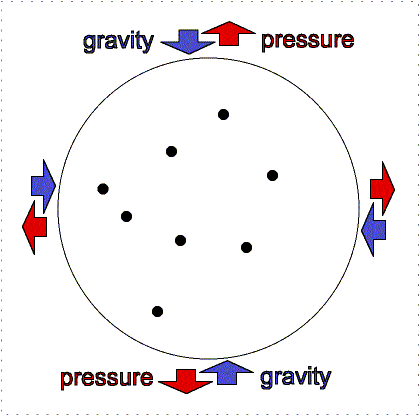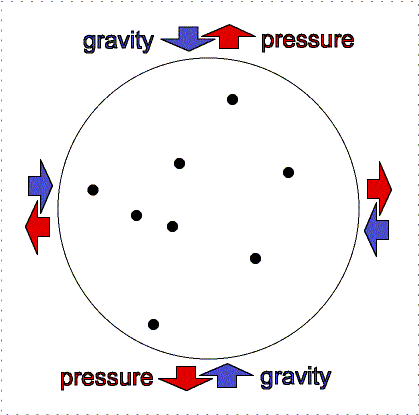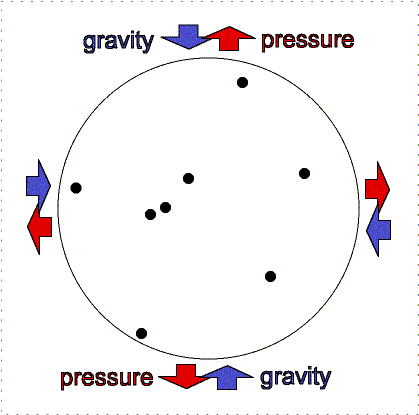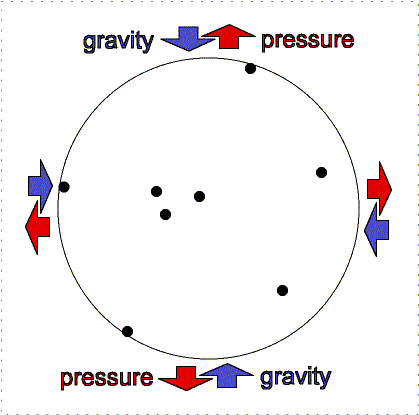
gravity compression is balanced by pressure outward
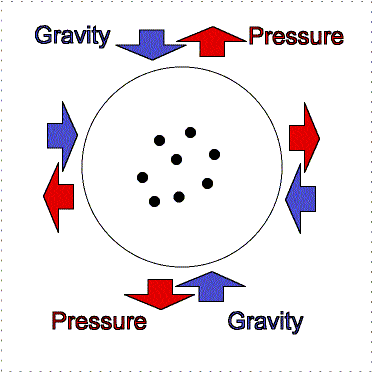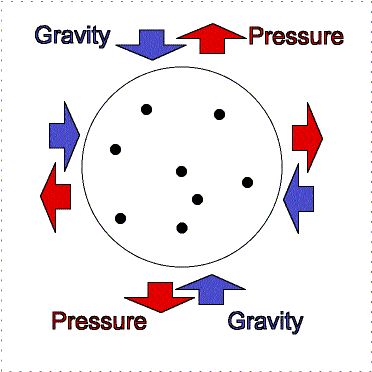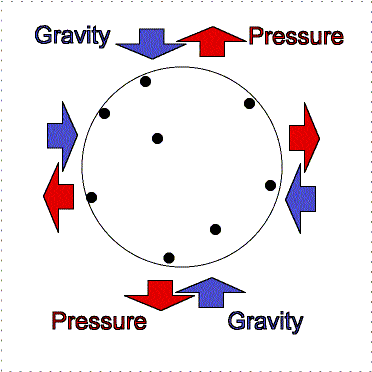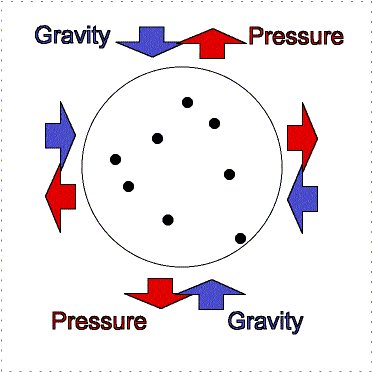
Greater gravity compresses the gas, making it denser and hotter, so the
outward pressure increases
- thermal equilibrium - the amount of energy generated in the core
of a star by thermonuclear fusion must equal the amount radiated away
(the only place for the energy to go is outward)

- opacity - how fast energy is radiated is determined by the
resistance of the stellar envelope to the flow of photons. If a star
has low opacity, it can radiate its energy fast and its temperature
and pressure will be lower = smaller radii
 At a star's surface the energy is released to form the spectrum of the
star.
At a star's surface the energy is released to form the spectrum of the
star.
- energy transport - how energy is transported from the core to the
stellar surface determines the surface temperature of a star (its
color)
There are three ways to transfer energy; conduction, convection and
radiation. Conduction, the collisional transfer of energy between
atoms, only occurs between solids (such as a hot pan and your hand),
so is not found in stars. Only convection and radiation transfer are
important in stars and the opacity determines which method is used.
When the temperature gradient is low and all the atoms are stripped of their
electrons, the opacity is low and radiation transfer is dominant.
 When the temperature drops, and the temerature gradient is high,
such as in the outer layers of a stars interior, the protons and
electrons recombine to form atoms and the opacity goes up. High
opacity slows the transfer of energy by radiation, so bubbles
form. These bubbles are hot and low in density, thus starting a
convective flow.
When the temperature drops, and the temerature gradient is high,
such as in the outer layers of a stars interior, the protons and
electrons recombine to form atoms and the opacity goes up. High
opacity slows the transfer of energy by radiation, so bubbles
form. These bubbles are hot and low in density, thus starting a
convective flow.
 Whether convection or radiation transport is used depends on the
temperature make-up of the stellar interior. When the changes in
temperature are sharp, convection is used. Think of the photons as
grains of sand on a pile. If the pile is low, radiation is used. If
the pile is high, the sand tumbles down, convection is used.
Whether convection or radiation transport is used depends on the
temperature make-up of the stellar interior. When the changes in
temperature are sharp, convection is used. Think of the photons as
grains of sand on a pile. If the pile is low, radiation is used. If
the pile is high, the sand tumbles down, convection is used.
At a star's surface the convective cells release energy, as shown in this
supercomputer movie.
- energy production - in the case of stars, energy is produced by
thermonuclear fusion. This can be either the proton-proton chain or
the CNO cycle.
These 5 relationships, stated as mathematical formula, show how
energy is generated, how that energy effects the structure of stars
and how that energy is transported to the surface to make a star
shine.
Stellar Interior:
A star is divided into six regions based on the physical
characteristics of these regions. The boundaries are not sharp, and
the regions vary in size from star to star. For example, hot stars
have larger radiative zones and smaller convective zones. The
reverse is true of cool stars.
- fusion core - region of energy generation
- radiation shell - the region where energy transport is by radiation
flow
- convection shell - the region where energy transport is by
convection cells
- photosphere - the surface where photons are emitted, where features
like starspots and stellar flares occur
- chromosphere - the atmosphere of a star
- corona - the super hot region where the stellar wind originates
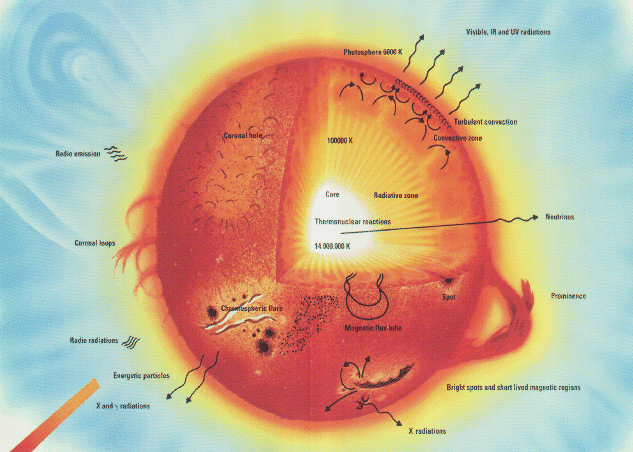 The radii and temperatures of these regions for a typical solar mass star
are the following:
The radii and temperatures of these regions for a typical solar mass star
are the following:
region radius temperature
-------------------------------------------------
fusion core 0.3 solar radii 15x10^6 K
radiation shell 0.3-0.6 solar radii 6x10^6 K
convection shell 0.6-1.0 solar radii 1x10^6 K
photosphere 100 km 6000 K
chromosphere 2000 km 30,000 K
corona 10^6 km 1x10^6 K
Stars rotates differentially since they are not solid. The stellar
equator for a solar mass star completes one rotation in 25 days. The poles complete one
rotation in 36 days.
Chromosphere of Stars:
The chromosphere is a pinkish atmosphere above a stars surface (the
photosphere). It emits an emission spectrum, which indicates it
chromosphere's are composed of a very hot gas (20,000 K or more).
The most complex and transient stellar phenomena occur in the
chromosphere including:
- stellar flares
- stellar arcs
- prominences
Flares, arcs and prominences are linked to stellar activity, very small
changes in stellar luminosity .
Gas is trapped in the flux lines created by the starspots and
lifted off the photosphere into the chromosphere. Over a period of a
few hours, the magnetic fields collapse hurling the hot gas outward
(much like a breaking rubber band).
Stellar Corona:
The corona of a star is a
large, white halo of glowing gas. The
corona gas is extremely hot (temperatures on order of a million
degrees) and is the source of the stellar winds.
A stellar
wind is a constant stream of sub-atomic particles moving at faster
than the escape velocity of the star's gravitational field. They
escape through windows in the corona, called coronal holes,
regions where the magnetic fields are weak and the charged stellar wind
particles are not trapped in magnetic bottles.
X-ray and UV pictures of the corona
show that the hot gas is connected to the magnetic features in the
photosphere. Those low level structures extending into long streamers in the outer corona and
heat the corona to its million degree temperatures.
Degeneracy:
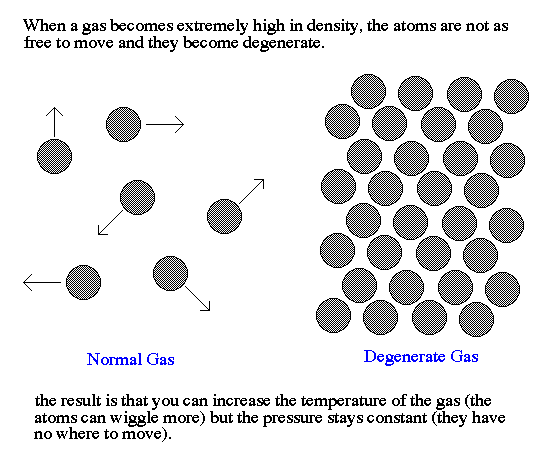
Gases normally obey the Ideal Gas Law. However, when the atoms
become very densely packed, special rules of quantum mechanics
control the behavior of the atoms. In particular, the Pauli exclusion
principle limits the number of particles of a given kind that can
be put into a cubic centimeter of space. When the density of the gas
reaches this limit it begins to behave like a liquid.
For normal gases, when the density or temperature go up, the pressure
goes up. For extremely high density gases, the pressure becomes almost
independent of temperature and the gas is said to be degenerate. This
happens for normal matter when the density reaches 1015 gm/cc
(for example, if you squeeze the whole Earth into a football stadium).
 The cores of normal stars are an ideal gas, and follow the ideal gas
law. However, as hydrogen fuel is burned, helium is produced as a
byproduct and begins to build up in the center of the star. Helium
cannot burn at these lower temperatures, so the helium ash becomes
denser and denser to reach degeneracy. The core can cool, but it
doesn't contract because its internal pressure remains high even as
its temperature falls. Thus, stars with low mass cores become white
dwarfs, slowly cooling and radiating their heat into space.
The cores of normal stars are an ideal gas, and follow the ideal gas
law. However, as hydrogen fuel is burned, helium is produced as a
byproduct and begins to build up in the center of the star. Helium
cannot burn at these lower temperatures, so the helium ash becomes
denser and denser to reach degeneracy. The core can cool, but it
doesn't contract because its internal pressure remains high even as
its temperature falls. Thus, stars with low mass cores become white
dwarfs, slowly cooling and radiating their heat into space.










