Electromagnetic radiation is energy that is propagated through free space or through a material medium in the form of electromagnetic waves, such as radio waves, visible light, and gamma rays. The term also refers to the emission and transmission of such radiant energy.
The Scottish physicist James Clerk Maxwell was the first to predict the existence of electromagnetic waves. In 1864 he set forth his electromagnetic theory, proposing that light--including various other forms of radiant energy--is an electromagnetic disturbance in the form of waves. In 1887 Heinrich Hertz, a German physicist, provided experimental confirmation by producing the first man-made electromagnetic waves and investigating their properties. Subsequent studies resulted in a broader understanding of the nature and origin of radiant energy.

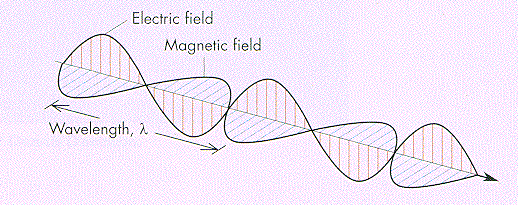
It has been established that time-varying electric fields can induce magnetic fields and that time-varying magnetic fields can in like manner induce electric fields. Because such electric and magnetic fields generate each other, they occur jointly, and together they propagate as electromagnetic waves. An electromagnetic wave is a transverse wave in that the electric field and the magnetic field at any point and time in the wave are perpendicular to each other as well as to the direction of propagation. In free space (i.e., a space that is absolutely devoid of matter and that experiences no intrusion from other fields or forces), electromagnetic waves always propagate with the same speed--that of light (299,792,458 m per second, or 186,282 miles per second)--independent of the speed of the observer or of the source of the waves.
Electromagnetic radiation has properties in common with other forms of waves such as reflection, refraction, diffraction, and interference. Moreover, it may be characterized by the frequency with which it varies over time or by its wavelength. Electromagnetic radiation, however, has particle-like properties in addition to those associated with wave motion. It is quantized in that for a given frequency , its energy occurs as an integer times h , in which h is a fundamental constant of nature known as Planck's constant. A quantum of electromagnetic energy is called a photon. Visible light and other forms of electromagnetic radiation may be thought of as a stream of photons, with photon energy directly proportional to frequency.
Electromagnetic radiation spans an enormous range of frequencies or wavelengths, as is shown by the electromagnetic spectrum. Customarily, it is designated by fields, waves, and particles in increasing magnitude of frequencies--radio waves, microwaves, infrared rays, visible light, ultraviolet light, X rays, and gamma rays. The corresponding wavelengths are inversely proportional, and both the frequency and wavelength scales are logarithmic.
Electromagnetic radiation of different frequencies interacts with matter differently. A vacuum is the only perfectly transparent medium, and all material media absorb strongly some regions of the electromagnetic spectrum. For example, molecular oxygen (O2), ozone (O3), and molecular nitrogen (N2) in the Earth's atmosphere are almost perfectly transparent to infrared rays of all frequencies, but they strongly absorb ultraviolet light, X rays, and gamma rays. The frequency (or energy equal to hv) of X rays is substantially higher than that of visible light, and so X rays are able to penetrate many materials that do not transmit light. Moreover, absorption of X rays by a molecular system can cause chemical reactions to occur. When X rays are absorbed in a gas, for instance, they eject photoelectrons from the gas, which in turn ionize its molecules. If these processes occur in living tissue, the photoelectrons emitted from the organic molecules destroy the cells of the tissue. Gamma rays, though generally of somewhat higher frequency than X rays, have basically the same nature. When the energy of gamma rays is absorbed in matter, its effect is virtually indistinguishable from the effect produced by X rays.
There are many sources of electromagnetic radiation, both natural and man-made. Radio waves, for example, are produced by cosmic objects such as pulsars and quasars and by electronic circuits. Sources of ultraviolet radiation include mercury vapour lamps and high-intensity lights, as well as the Sun. The latter also generates X rays, as do certain types of particle accelerators and electronic devices.
Excerpt from the Encyclopedia Britannica without permission.