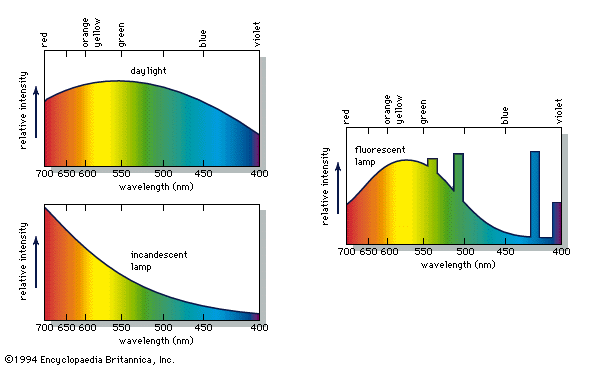
The energy distribution in light from daylight, an incandescent lamp, and a fluorescent lamp.
Light, a basic aspect of the human environment, cannot be defined in terms of anything simpler or more directly appreciated by the senses than itself. Light, certainly, is responsible for the sensation of sight. Light is propagated with a speed that is high but not infinitely high. Physicists are acquainted with two methods of propagation from one place to another, as (1) particles and as (2) waves, and for a long time they have sought to define light in terms of either particles or waves. In the early 19th century a wave description was favoured, though it was difficult to understand what kind of wave could possibly be propagated across the near-vacuum of interstellar space and with the extremely high speed of 300,000 kilometers per second (186,000 miles per second). In the latter half of the 19th century a British physicist, James Clerk Maxwell, showed that certain electromagnetic effects could be propagated through a vacuum with a speed equal to the measured speed of light. Thus, in the second half of the 19th century, light was described as electromagnetic waves. Such waves were visualized as analogous to those on the surface of water (transverse waves) but with an extremely short wavelength of about 500 nanometers (one nanometer is 10-9 meter). The analogy is valid up to a certain point but the experimental results obtained at the end of the 19th century and in the early years of the 20th century revealed properties of light that could not have been predicted from knowledge that was obtainable about other waves. These results led to the quantum theory of light, which in its primitive form asserted that, at least in regard to its emission and absorption by matter, light behaves like particles rather than waves. The results of certain important experiments on the spreading of light into shadows and other experiments (on the interaction of beams of light) that supported the wave theory found no place in a particle theory. For a time it was believed that light could not be adequately described by analogy with either waves or particles--that it could be defined only by a description of its properties. A reconciliation of wave and particle concepts did not emerge until after 1924.
Two properties of light are, perhaps, more basic and fundamental than any others. The first of these is that light is a form of energy conveyed through empty space at high velocity (in contrast, many forms of energy, such as the chemical energy stored in coal or oil, can be transferred from one place to another only by transporting the matter in which the energy is stored). The unique property of light is, thus, that energy in the form of light is always moving, and its movement is only in an indirect way affected by motion of the matter through which it is moving. (When light energy ceases to move, because it has been absorbed by matter, it is no longer light.)
The second fundamental property is that a beam of light can convey information from one place to another. This information concerns both the source of light and also any objects that have partly absorbed or reflected or refracted the light before it reaches the observer. More information reaches the human brain through the eyes than through any other sense organ. Even so, the visual system extracts only a minute fraction of the information that is imprinted on the light that enters the eye. Optical instruments extract much more information from the visual scene; spectroscopic instruments, for example, reveal far more about a source of light than the eye can discover by noting its colour, and telescopes and microscopes extract scientific information from the environment. Modern optical instruments produce, indeed, so much information that automatic methods of recording and analysis are needed to enable the brain to comprehend it.
Electromagnetic radiation spans an enormous range of frequencies or wavelengths, as is shown by the electromagnetic spectrum. Customarily, it is designated by fields, waves, and particles in increasing magnitude of frequencies--radio waves, microwaves, infrared rays, visible light, ultraviolet light, X rays, and gamma rays. The corresponding wavelengths are inversely proportional, and both the frequency and wavelength scales are logarithmic.

The energy distribution in light from daylight, an incandescent lamp, and a fluorescent lamp.
Visible light is the most familiar form of electromagnetic radiation and makes up that portion of the spectrum to which the eye is sensitive. This span is very narrow; the frequencies of violet light are only about twice those of red. The corresponding wavelengths extend from 7 10-5 centimeter (red) to 4 10-5 centimeter (violet). The energy of a photon from the centre of the visible spectrum (yellow) is h = 2.2 eV. This is one million times larger than the energy of a photon of a television wave and one billion times larger than that of radio waves in general.
Excerpt from the Encyclopedia Britannica without permission.