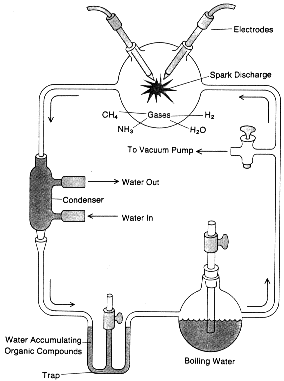
The first deliberate experimental simulation of these primitive conditions was carried out in 1953 by a U.S. graduate student, S.L. Miller, under the guidance of the eminent chemist H.C. Urey. A mixture of methane, ammonia, water vapour, and hydrogen was circulated through a liquid water solution and continuously sparked by a corona discharge elsewhere in the apparatus. The discharge may be thought to represent lightning flashes on the early Earth. After several days of exposure to sparking, the solution changed color. Subsequent analysis indicated that several amino and hydroxy acids, intimately involved in contemporary life, had been produced by this simple procedure. The experiment is in fact so elementary, and the amino acids can so readily be detected by paper chromatography, that the experiment has been repeated many times by high school students. Subsequent experiments have substituted ultraviolet light or heat as the energy source or have altered the initial abundances of gases. In all such experiments amino acids have been formed in large yield. On the early Earth there was much more energy available in ultraviolet light than in lightning discharges. At long ultraviolet wavelengths, in which methane, ammonia, water, and hydrogen are all transparent, but in which the bulk of the solar ultraviolet energy lies, the gas hydrogen sulfide (H2S) is a likely ultraviolet absorber.

Following such reasoning, a U.S. astrophysicist, Carl Sagan, and his colleagues made amino acids by long wavelength ultraviolet irradiation of a mixture of methane, ammonia, water, and H2S. The amino acid syntheses, at least in many cases, involve hydrogen cyanide and aldehydes (e.g., formaldehyde) as gaseous intermediaries formed from the initial gases. It is quite remarkable that amino acids, particularly biologically abundant amino acids, can be made so readily under simulated primitive conditions. When laboratory conditions become oxidizing, however, no amino acids are formed, suggesting that reducing conditions were necessary for prebiological organic synthesis.
Under alkaline conditions, and in the presence of inorganic catalysts, formaldehyde spontaneously reacts to form a variety of sugars, including the five-carbon sugars fundamental to the formation of nucleic acids and such six-carbon sugars as glucose and fructose, which are extremely common metabolites and structural building blocks in contemporary organisms. Furthermore, the nucleotide bases as well as porphyrins have been produced in the laboratory under simulated primitive Earth conditions by several investigators. While there is still debate on the generality of the experimental synthetic pathways and on the stability of the molecules produced, most if not all of the essential building blocks of proteins, carbohydrates, and nucleic acids can be readily produced under quite general primitive reducing conditions.
Excerpt from the Encyclopedia Britannica without permission.