The quantum theory of absorption and emission of radiation announced in 1900 by Planck ushered in the era of modern physics. He proposed that all material systems can absorb or give off electromagnetic radiation only in "chunks" of energy, quanta E, and that these are proportional to the frequency of that radiation E = h. (The constant of proportionality h is, as noted above, called Planck's constant.)
Planck was led to this radically new insight by trying to explain the puzzling observation of the amount of electromagnetic radiation emitted by a hot body and, in particular, the dependence of the intensity of this incandescent radiation on temperature and on frequency. The quantitative aspects of the incandescent radiation constitute the radiation laws.
The Austrian physicist Josef Stefan found in 1879 that the total radiation energy per unit time emitted by a heated surface per unit area increases as the fourth power of its absolute temperature T (Kelvin scale). This means that the Sun's surface, which is at T = 6,000 K, radiates per unit area (6,000/300)4 = 204 = 160,000 times more electromagnetic energy than does the same area of the Earth's surface, which is taken to be T = 300 K. In 1889 another Austrian physicist, Ludwig Boltzmann, used the second law of thermodynamics to derive this temperature dependence for an ideal substance that emits and absorbs all frequencies. Such an object that absorbs light of all colours looks black, and so was called a blackbody.

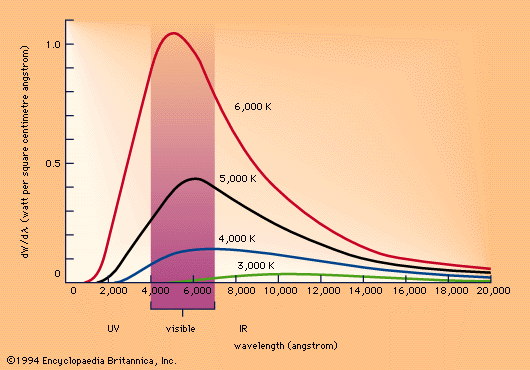
The wavelength or frequency distribution of blackbody radiation was studied in the 1890s by Wilhelm Wien of Germany. It was his idea to use as a good approximation for the ideal blackbody an oven with a small hole. Any radiation that enters the small hole is scattered and reflected from the inner walls of the oven so often that nearly all incoming radiation is absorbed and the chance of some of it finding its way out of the hole again can be made exceedingly small. The radiation coming out of this hole is then very close to the equilibrium blackbody electromagnetic radiation corresponding to the oven temperature. Wien found that the radiative energy per wavelength interval has a maximum at a certain wavelength and that the maximum shifts to shorter wavelengths as the temperature T is increased, as illustrated in the above figure.
Wien's law of the shift of the radiative power maximum to higher frequencies as the temperature is raised expresses in a quantitative form commonplace observations. Warm objects emit infrared radiation, which is felt by the skin; near T = 950 K a dull red glow can be observed; and the colour brightens to orange and yellow as the temperature is raised. The tungsten filament of a light bulb is T = 2,500 K hot and emits bright light, yet the peak of its spectrum is still in the infrared according to Wien's law. The peak shifts to the visible yellow when the temperature is T = 6,000 K, like that of the Sun's surface.
It was the shape of Wien's radiative energy distribution as a function of frequency that Planck tried to understand. The decrease of the radiation output at low frequency had already been explained by Lord Rayleigh (John William Strutt) in terms of the decrease, with lowering frequency, in the number of modes of electromagnetic radiation per frequency interval. Rayleigh assumed that all possible frequency modes could radiate with equal probability, following the principle of equipartition of energy. Since the number of frequency modes per frequency interval continues to increase without limit with the square of the frequency, Rayleigh's formula predicted an ever-increasing amount of radiation of higher frequencies instead of the observed maximum and subsequent fall in radiative power. A possible way out of this dilemma was to deny the high-frequency modes an equal chance to radiate. To achieve this, Planck postulated that the radiators or oscillators can only emit electromagnetic radiation in finite amounts of energy of size. At a given temperature T, there is then not enough thermal energy available to create and emit many large radiation quanta. More large energy quanta can be emitted, however, when the temperature is raised.
Excerpt from the Encyclopedia Britannica without permission.