

Spectra of Light :
Spectrum, in optics, the arrangement according to wavelength of visible, ultraviolet, and infrared light. An instrument designed for visual observation of spectra is called a spectroscope; an instrument that photographs or maps spectra is a spectrograph. The typical spectroscope is a combination of a microscope and a prism. The prism breaks the light into its spectra components (by differential refraction) which is then magnified with a microscope.
Spectra may be classified according to the nature of their origin, i.e., emission or absorption. An emission spectrum consists of all the radiations emitted by atoms or molecules, whereas in an absorption spectrum, portions of a continuous spectrum (light containing all wavelengths) are missing because they have been absorbed by the medium through which the light has passed; the missing wavelengths appear as dark lines or gaps.
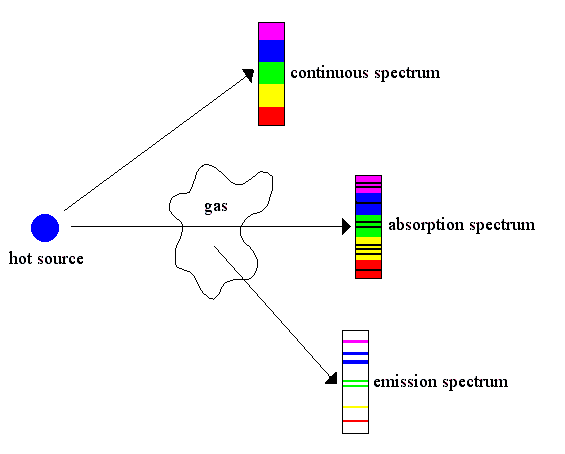
The spectrum of incandescent solids is said to be continuous because all wavelengths are present. The spectrum of incandescent gases, on the other hand, is called a line or emission spectrum because only a few wavelengths are emitted. These wavelengths appear to be a series of parallel lines because a slit is used as the light-imaging device. Line spectra are characteristic of the elements that emit the radiation. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from one energy level to another. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band, e.g., nitrogen spectrum. Band spectra, or molecular spectra, are produced by molecules radiating their rotational or vibrational energies, or both simultaneously.
Excerpt from the Encyclopedia Britannica without permission.