The atoms fly about and bump into each other. The hotter the gas is, the faster the average speed of the atoms is.
Inside matter the atoms (or molecules) are always moving. For instance,
a gas (air, inside the Sun ...) might look something like this

The atoms fly about and bump into each other.
The hotter the gas is, the faster the average speed of the atoms is.
As the atoms move and change directions, they emit electromagnetic radiation.
Sometimes you can see this radiation, somethimes you can't. The inside of a kiln used for melting glass to make beads and other small decorations is an approximate example.
There is an ideal case of this, called blackbody radiation. Many real cases of warm or hot bodies emitting radiation are pretty close to this ideal case.
Blackbody radiation is emitted over a range of wavelengths.
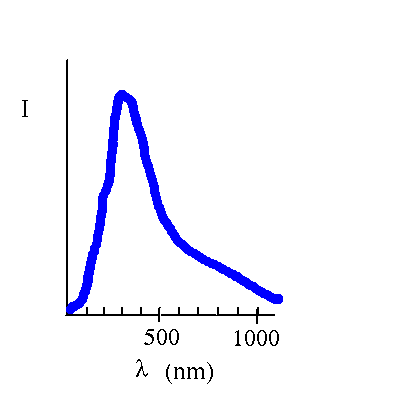
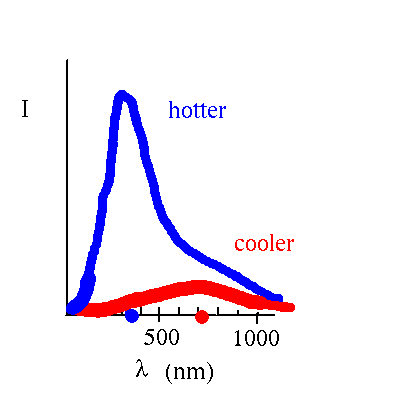
The amount of radiation and the distribution as a function of wavelength depends on the temperature (but not on the material in the ideal case of blackbody radiation)
The quantitative relations are
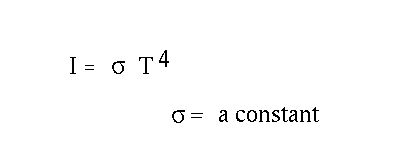
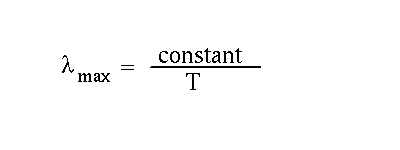
We will use the T4 law later. For now, concentrate on how the wavelength varies with temperature. It gives us important information on the question, " How hot is the Sun?"
Updated 1 October 2007
Davison E. Soper, Institute of Theoretical Science, University of Oregon, Eugene OR 97403 USA