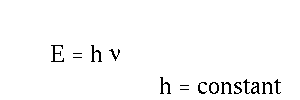
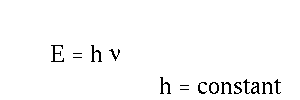
Here h is called Plank's constant and is very tiny.
The energy of any one photon is tiny compared to any "macroscopic" energy scale. Thus there are zillions of photons in an ordinary light beam.
But for frequencies as in visible light or for larger frequencies, the energy of a single photon is not so small compared to energies of single atoms.
The photon concept is important because photons are emitted and absorbed one photon at a time. For instance, a molecule in a vision cell in your eye can absorb one photon, but never half a photon.
For frequencies of xrays and above, the energy of a single photon is large compared to the energies typical of atoms. That is why a single xray photon can shoot right through your body.
The "discovery" of the photon, based on the interpretation of experimental results that otherwise seemed mysterious, was made in a scientific paper by Albert Einstein in 1905.
Davison E. Soper, Institute of Theoretical Science, University of Oregon, Eugene OR 97403 USA soper@bovine.uoregon.edu