Fusion in the Sun step by step
The overall fusion reaction
4 1H + 2 e --> 4He + 2 neutrinos + 6 photons
happens in several steps. (This is not different from methane
burning, which also happens in steps.)
Step 1
1H + 1H --> 2H + antielectron + neutrino
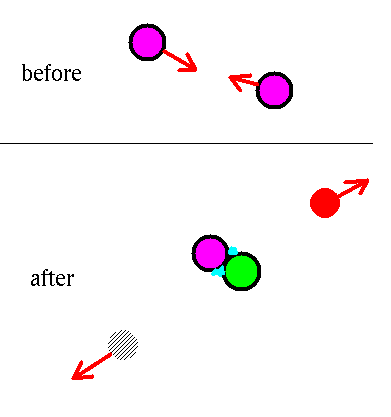
This step is nearly impossible.
- The protons repel each other because they both have + 1 charge.
- They have to get within 10-15 m of each other for
the strong interactions to hold them together.
- One of the protons has to change into a neutron (emitting
an anti-electron and neutrino).
- This requires a weak interaction.
- But the chance of this weak interaction happening just when
the protons are together is almost zero.
This near impossiblity has two consequences:
- The gas must be very hot, so that the protons hit each other
with high speed.
- This lets them get near to each other, even though they repel
each other.
- Even so, the reaction is very rare.
That is why the Sun is still burning after 4.6 billion years!
Step 2
electron + antielectron --> photon + photon
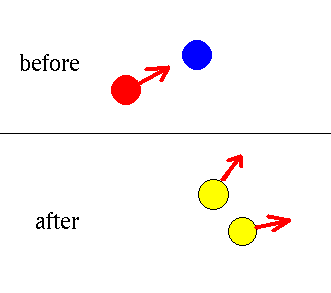
The antielectron soon hits an electron. They annihilate to
make two photons. (These high energy photons will be absorbed in
the gas, eventually giving lots of low energy photons.)
Step 3
2H + 1H --> 3He + photon
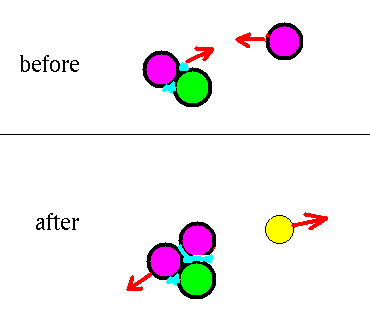
The 2H hits a proton and sticks to it, making a
3He and emitting a photon in the process.
(This high energy photon will be absorbed in
the gas, eventually giving lots of low energy photons.)
Step 4
3He + 3He --> 4He
+ 1H+ 1H
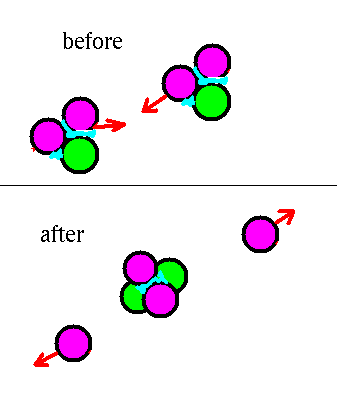
Since these reactions make 3He nuclei, there are
some of these bouncing around in the gas. When two of them
hit each other, the 2 neutrons and 4 protons of
rearrange themselves into one 4He nucleus and
two free protons.
Note that steps 1,2 and 3 each happen twice for each
time step 4 happens.
The net result is
1H + 1H --> 2H + antielectron + neutrino
1H + 1H --> 2H + antielectron + neutrino
electron + antielectron --> photon + photon
electron + antielectron --> photon + photon
2H + 1H --> 3He + photon
2H + 1H --> 3He + photon
3He + 3He --> 4He
+ 1H+ 1H
That is
6 1H + 2 e --> 4He + 2 1H
+ 2 neutrinos + 6 photons
or
4 1H + 2 e --> 4He
+ 2 neutrinos + 6 photons
The amount of energy released in each separate reaction has been
measured. The net energy release is 26 MeV.
There are some other reactions also. They are less significant
because they don't produce much energy. [For this reason, please
don't try to memorize them.] But they have a special significance
because they produce neutrinos, which we will study.
- 3He + 4He --> 7Be + photon
- 7Be + electron --> 7Li + neutrino
- 7Li + 1H --> 2 4He
- alternatively
- 7Be + 1H --> 8B + photon
- 8B --> 2 4He + antielectron + neutrino
There is another energy producing cycle that is important in
hot stars (temperatures bigger than 16 x 106K).
It is called the CNO cycle. In it, carbon is a catalyst. It
is needed to make the reaction work, but is not used up. [Again, I
would suggest that you not memorize the cycle, but do understand
the concept of a catalyst, which is important in practical chemical
reactions.]
- start with 12C
- 12C + 1H --> 13N + photon
- 13N --> 13C + antielectron + neutrino
- 13C + 1H --> 14N + photon
- 14N + 1H --> 15O + photon
- 15O --> 15N + antielectron + neutrino
- 15N + 1H --> 12C + 4He
The net result is that you get the 12C back and you have
used up 4 protons to make one 4He, along with two antielectrons
and three photons. The antielectrons will annihilate with electrons to
make photons.
So hydrogen is burned to make helium with carbon as a catalyst.
Davison E. Soper, Institute of Theoretical Science,
University of Oregon, Eugene OR 97403 USA
soper@bovine.uoregon.edu




