Homestake gold mine experiment
The pioneering experiment in this direction was performed deep in the
Homestake Gold Mine in South Dakota starting in the early 1970's. It was organized by a chemist, Ray Davis.
The experiment is deep underground to protect it from high energy
particles from outer space called cosmic rays. The
detection method was based on the reaction
37Cl + neutrino --> 37Ar + electron.
(Chlorine, Cl has 17 protons while argon, Ar has 18 protons. Thus one
neutron got converted into a proton.)
After a few days, the argon decays back to chlorine:
37Ar --> 37Cl + neutrino + antielectron .
The idea is to tell that the reaction happened by seeing the antielectron.
It's not so easy. Neutrios interact so infrequently that you need to
have a lot of chlorine, and then expect to make one argon atom per day.
Here is a picture of how it works.
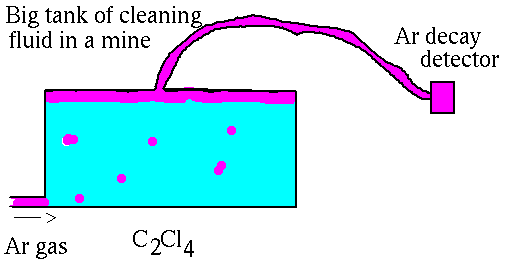
This experiment ran for many years. The first results were announced in the early 1970s. The result was that about 1/3 of the expected number of reactions occurred. This suggested three possibilities
- The experiment was wrong.
- The standard solar model was wrong.
- It would be hard to get the number of neutrinos from p + p --> 2H + electron + neutrino wrong because this is the first step in the main energy producing reaction of the sun and we know how much energy is produced.
- The experiment was not capable of seeing the p + p --> 2H + electron + neutrino because most of the neutrinos produced have too
low energy to make the chlorine reaction occur.
- The neutrinos that it could see result from a rare reaction in the sun.
- It would be pretty easy to get the rate for this reaction wrong.
- The standard picture of neutrinos was wrong. Electron neutrinos could oscillate to become muon neutrinos, which don't interact with chlorine.
At the time, it seemed that explanations 1 or 2 were most likely.
ASTR 122 course home page
Updated 22 Octobber 2007
Davison E. Soper, Institute of Theoretical Science,
University of Oregon, Eugene OR 97403 USA

