Pieter Bruegel the Elder (1525-1569)
|
Why Do We Study Stars?
- Stars are interesting
- Stars are very luminous, LSun = 4x1033erg/s
- indirect energy source
- direct energy source
- Solar-Terrestrial climate connections
- Stars are High-Energy Physics Laboratories
- Stars are useful probes of the properties of the Universe
- ....
|
THE SUN (and stars) ARE PRODIGIOUS SOURCES OF ENERGY
 |
World-Wide Energy Consumption
1 TW (terraWatt) = 1 TWatt = 109 kWatt (kiloWatt)
===>
10 TW = 2.5x10-14 of the Solar luminosity (power output)!
Note that out of the energy sources listed, four (oil, coal, gas, hydro)
are from the Sun. The only one which is not is nuclear.
Note:
What's a Watt? A Watt is an energy expenditure of 1 Joule per second.
Okay, so what
is a Joule? Let's see, mosquitos are 1-2 milligrams (~2-4 millionths
of a pound) and fly at speeds of ~1-2 km per hour. So, a flying
mosquito has kinetic energy ~ 4x10-7 Joule,
or a swarm of 2.5 million
mosquitos carries kinetic energy of ~1 Joule! |
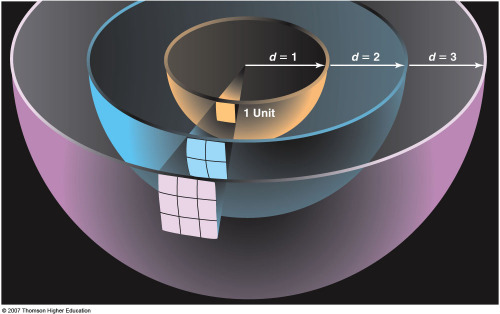 |
There is more to the story than this, however. Because of the large distance
to the Sun (150,000,000 km), we intercept only ~2.2x10-5 of the
Sun's power per unit area (its energy Flux).
The brightness of the Sun (its flux) falls off as 1/D2,
where D is the distance to the Sun. Although the amount of energy we
intercept because of this effect (the inverse square fall-off of the
brightness of the Sun) is tiny, the fraction of the Solar power we
absorb is large in the sense that the Earth easily intercepts enough
energy from the Sun to satisfy our energy needs. Even allowing for cloud
cover (Albedo effects) and the absorption of light in our atmosphere
(our atmosphere is not transparent because of opacity effects), the
energy which reaches the ground is substantial, ~0.34 kWatts per square meter,
A Solar collector ~100 miles x 100 miles in size is capable of capturing enough
Solar energy to satisfy the current energy needs of the Earth.
|
SOLAR-TERRESTRIAL CONNNECTIONS
 |
Sunspot Cycle and Solar Activity
The Sun exhibits cool blemishes on its surface known as
Sunspots. The average temperature of the surface of the Sun is
~5,800 Kelvin, Sunspots are ~4,500 Kelvin. Their lower temperatures
makes Sunspots appear darker than the surrounding regions of the
Sun (see comments after Stefan-Boltzmann Law [Lecture 3]. Sunspots were
discovered by Galileo in the 1600s. In and of themselves, Sunspots
are not that significant; they are symptomatic of the activity
of the Sun, however. |
 |
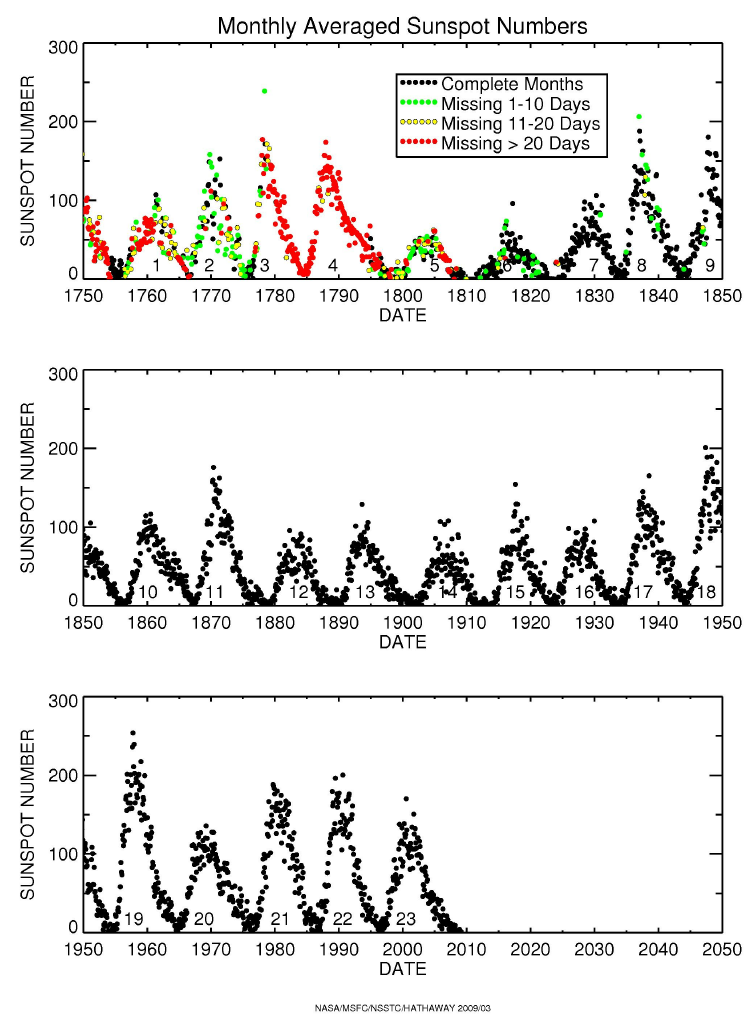 |
Sunspot Number
The Sun goes through an activity cycle, The Solar Activity
Cycle with the most obvious manifestation of the varying
number of Sunspots on its surface. The number varies with a period of
7-15 years with an average length of 11 years.
There are other effects which we shall describe later, such
as increases in coronal activity, increases in flaring activity, increases in
the Solar Wind, and increases in magnetic activity.
The cycle is fairly regular having been traced back hundreds
of years using tree ring studies and nearly 2,000 years using coral reefs.
Although regular, the Sunspot cycle
has shown disruptions. For example in 1645-1715, the cycle may have
halted during what is known as the
Maunder Minimum. Interestingly, at this time,
Northern Europe and North America were in the middle of what is referred to
as the Little Ice Age
).
There has been a recent suggestion that we may soon enter another
extended Solar Activity mininum (similar to the Dalton Minimum or
perhaps another Maunder Minimum). To see further information, go to
Solar Physics
Division Sunpot Cycle Release. |
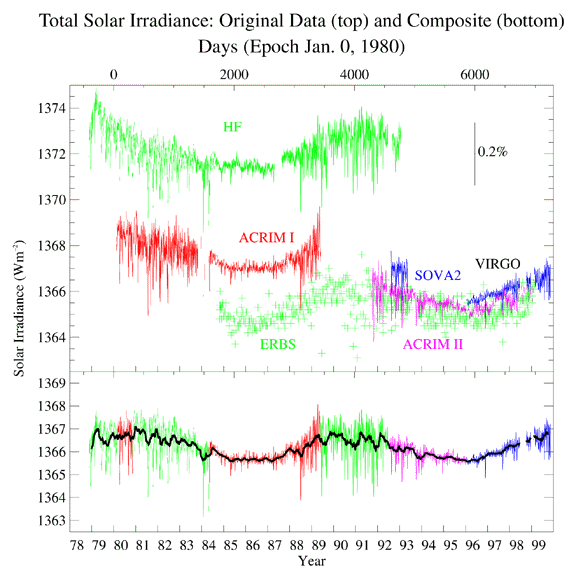 | Solar Constant
The vexing thing is that although there are measurable changes in the Solar
output during the Solar Activity Cycle, the change in the Solar
luminosity (as measured by the Solar Constant, see the figure to
the left) is small. The Solar Constant varies over the course
of the Solar Activity Cycle from
1,367 to 1,365 Watts per square meter, as measured at the top of the Earth's
atmosphere. The Sun (somewhat paradoxically) is the brightest at the peak of
the Solar Activity Cycle, when the greatest number of sunspots are seen.
|

| Faint Young Sun Paradox
The luminosity of the Sun
has increased as it has aged; 3.8 billion years ago the Sun
was ~25 % fainter than today.
This is a conundrum because there was liquid water on the
Earth at least 3.7 billion years ago and a
simple argument leads to a prediction
for what is referred to as the Equilibrium Temperature,
Te for the Earth which at that time, would be below the
freezing point of water, Te = -40 C!. Note that
Te is determined by simply finding the temperature for the Earth
where it radiates exactly the same amount of energy per second as it receives
from the Sun in the absence of clouds and an atmosphere. Further, if we were
to include an atmosphere with the composition of our current atmosphere, the
temperature would rise but would still be less than the freezing point of
water.
|
The answer to the question of then, why do we have liquid oceans?
requires that our atmosphere in the past had a much
different chemical composition than today
so that the Greenhouse Effect could maintain liquid oceans or, perhaps, the Sun
was much brighter in the past than we now believe.
SOLAR NEUTRINOS
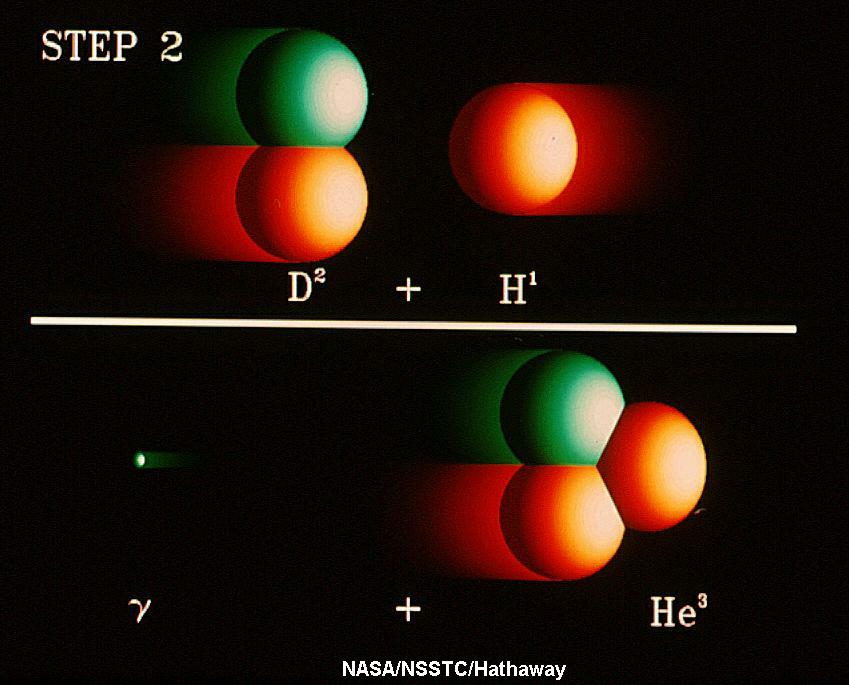
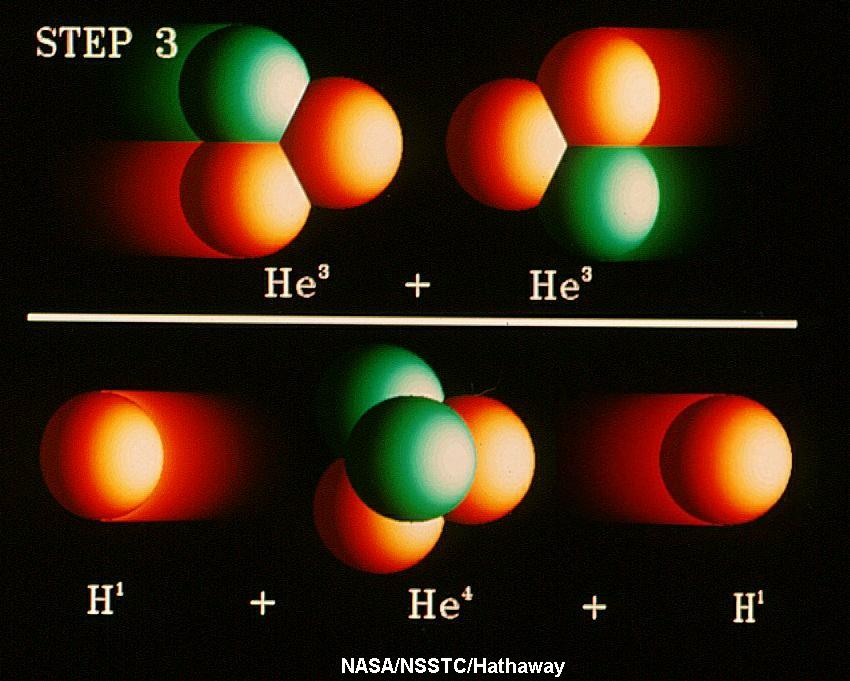
The Sun and normal stars are powered by nuclear fusion
reactions. In the Sun, the energy is produced through the Proton-Proton
Chain, the fusion of four hydrogen
nuclei into a helium nucles, plus some other fundamental particles, and
energy. As
a byproduct of the proton-proton chain reactions,
a ghostlike
particle known as the neutrino is also produced; several are
produced every time an energy generating
reaction occurs. Thus, if we truly understand how the Sun (and stars)
shine, we expect that a certain number of neutrinos must also
be produced and so, if we design an experiment to detect Solar
Neutrinos it must succeed in that it must detect the appropriate number
of neutrinos. There is no wiggle-room (or so we thought in the 1960s).
 |
Solar Neutrino
experiments were started in the 1960s by Brookhaven scientist, Ray Davis
to verify
that we understood how the Sun worked. No one thought that the experiment
would that interesting; it would be difficult but the result would not
be surprising. It came as a rude surprise when
Davis's experiment detected fewer neutrinos than predicted by the
best models of the Sun, throwing doubt onto whether we really did understand
our Sun. Follow-up experiments also found
~1/3-1/2 of predicted neutrinos. This conundrum
persisted for ~35 years until the early 2000s when, first,
the Super-K (Super Kamiokande) experiment showed
neutrinos were chamaeleon-like in nature.
Neutrinos, once produced, could change into forms
undetectable by the early experiments. The SNO
(Sudbury Neutrino Observatory) experiment,
able to detect transmuted neutrinos, then came online and
detected the predicted number of Solar neutrinos. The amusing result was
that a simple observation of the Sun led us to a deeper understanding of
how the Universe works on the sub-nuclear scale! |
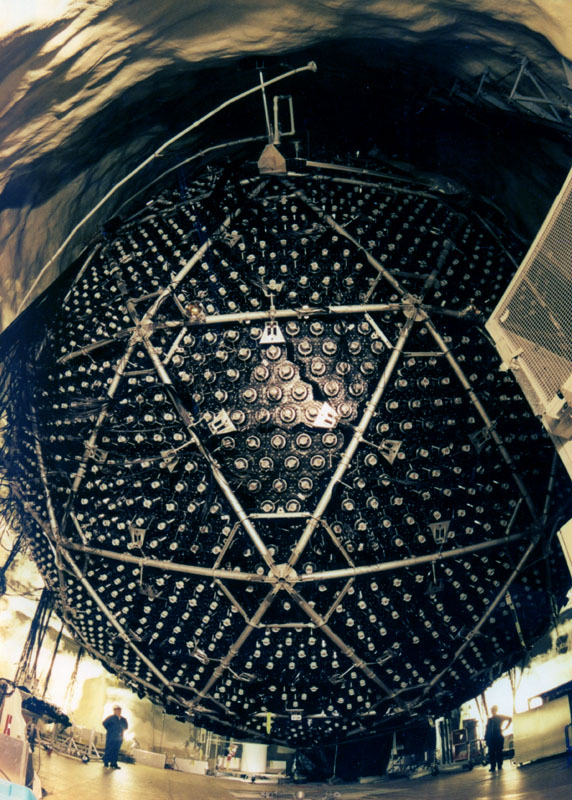 |
Comment: The Davis experiment was a remarkably difficult experiment. The
vat contained ~400,000 liters of cleaning fluid (tetrachloroethylene). This
vat contains ~2x1030 chlorine atoms. The neutrinos interact with
the chlorine (on rare occasions) transforming the chlorine to radioactive
argon which is subsequently detected.
A huge number of neutrinos passes through the vat every second, 400 billion
neutrinos flow through the detector per square inch per second. Remarkably,
one expects to build up only a few tens of Argon atoms every month!
This exceedingly difficult experiment was performed accurately enough by
Davis to show that there were roughly 1/3 the number of neutrinos passing
through his experiment as was predicted. Davis received the Nobel Prize
(along with Dr. Koshiba of the Super-K experiment) in
Physics in 2002 for this remarkable work.
WE ARE STARDUST

|
Big Bang, Solar, and Terrestrial Chemical Abundances
At left is shown the chemical make-up of the Sun.
In terms of the number of atoms, the
Sun is ~91 % hydrogen, ~8.9 % helium, and a little bit of everything
else. Also, more interestingly, when the Universe began, Big Bang created
primarily hydrogen and helium with essentially nothing heavier. How does this
compare to the Earth? Well, the
chemical abundance of the Earth is groslly different.
Most of the elements found in the Earth had to have been at one point in the
interior of a star. The heavy elements of which we are made were, for the most
part, produced in stars through fusion reactions.
|