EVOLUTION OF THE ATMOSPHERES OF THE TERRESTRIAL PLANETS
|

|
We consider:
We first address the atmospheric evolution of the Earth. We then
address the question of why the atmospheres of Mars and Venus
are so different. We finally look at the important question of
the evolution of oxygen in the atmosphere of the Earth.
I. ATMOSPHERIC EVOLUTION: EARTH, MARS, VENUS
Terrestrial planets (the atmosphere ones) are roughly
the same sizes and same distances from the Sun and yet, they have grossly
different kinds of atmospheres and conditions on their surfaces. Do we have
any ideas as to what leads to the huge differences? Surprisingly, there
may be simple explanations.
In the beginning, we believe that the material which was outgassed
from the interiors or carried in by comets or asteroids
onto the Terrestrial planets was similar. That is, the
Terrestrial planets started out roughly the same.
Originally what they were composed of depends
a little on their origins, but
they were likely dominated by
water and carbon dioxide, with varying amounts of sulfur dioxide,
carbon monoxide, nitrogen, and some other molecules and elements mixed in.
On each of Venus, Earth and Mars,
liquid oceans likely formed initially. On the Earth,
oceans formed in the Early Archean
period (the time before 2.5 billion years ago perhaps as long
ago as 4 billion years). Despite all having started with
oceans only the Earth has retained extensive oceans.
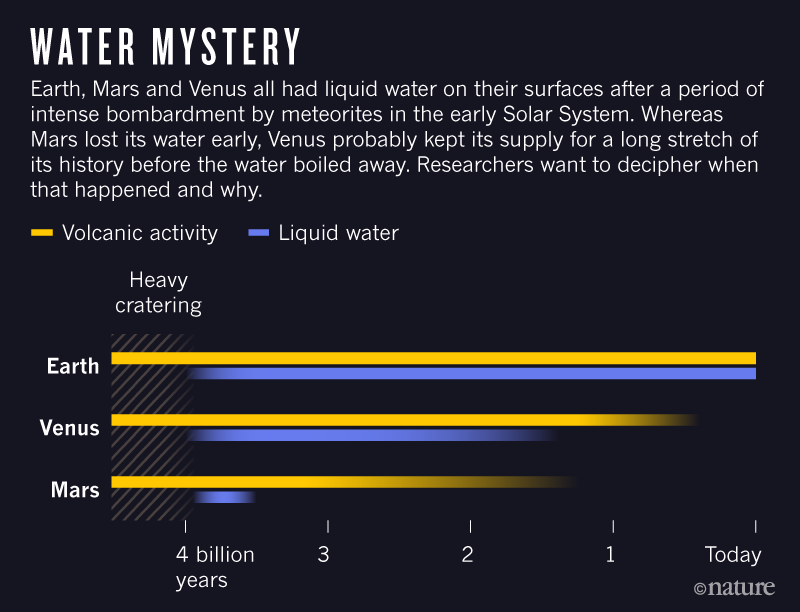
What caused the differences in the
evolution of the atmospheres of the Terrestrial planets
and liquid oceans as shown above?
On Venus, Earth and Mars,
- carbon dioxide
initially dissolved into the oceans, was rained
out of the atmosphere forming carbonic acid
which interacted with the surface silicate rocks producing calcium and
other ions which were washed into the oceans,
or was directly adsorded into the
rocks and washed into the oceans. (The first two processes are more efficient
at higher temperatures. weathering, the second process, is referred
to as silicate weathering
and plays a key role in regulating our Greenhouse effect.)
- Carbon dioxide
deposited into the oceans in this manner, settled
and formed sedimentary rocks ===> carbon dioxide was trapped
in the crust!
This happened fairly quickly on Earth
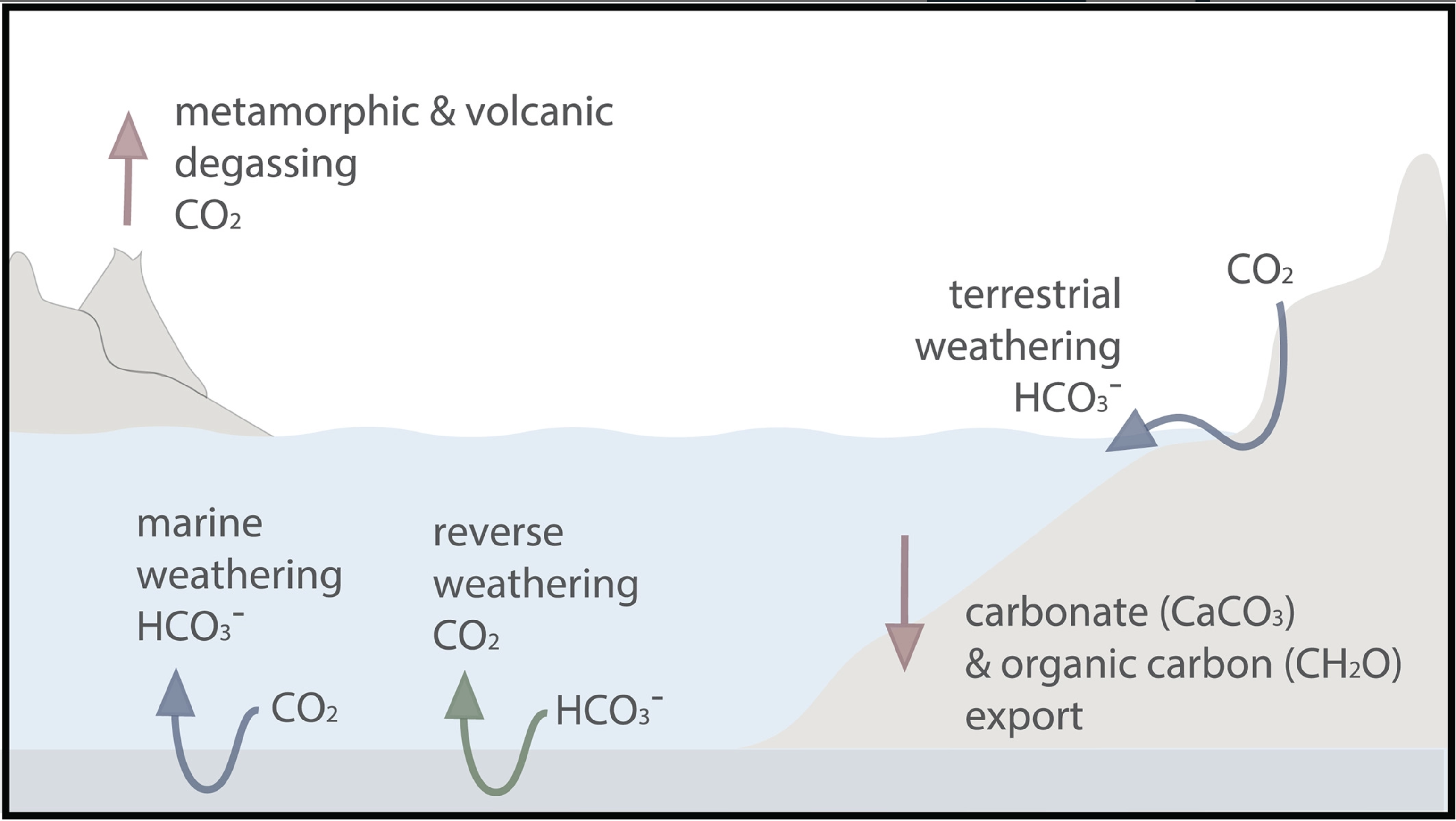
|
After this initial start-up, the evolutionary paths of Venus, Earth, and Mars
then diverged.
Earth: Recall that if the atmospheres of the early Terrestrial
planets had
no Greenhouse effect,
which would happen if they lost all of their carbon dioxide,
they would not have liquid oceans.
In order for the Earth to maintain its Greenhouse effect,
there must be a way for the Earth to return the carbon dioxide
taken from its atmosphere and trapped in its crust.
On the Earth, this happens
because we have plate tectonics. Carbon dioxide is liberated from the
crust and returned to the atmosphere through the volcanoes that form
near subduction zones. Without plate tectonics we would not be
able to maintain our temperate climate.
What happens as the Sun continues to get brighter as
it ages? This is an interesting question as the Earth has a
thermostat which can control its temperature.
As the Sun's brightness increases, we expect that the Earth's temperature
also rises. This is indeed true, but something interesting happens. As the
temperature goes up, the silicate weathering
process increases
and the biomass
increases both tending to remove more carbon dioxide
from the atmosphere and so to weaken the
Greenhouse effect and keep the Earth temperate. This control works until
all of the carbon dioxide is extracted from the atmosphere. At this point,
there is nothing to control the temperature, and so it
will continue to rise eventually causing
the oceans to start to evaporate. The evaporation leads to more water
vapor which leads to an enhanced
Greenhouse effect which leads to a higher temperature which
leads to more evaporation which leads to more water vapor and so on. This
positive feedback loop
leads to a Runaway Greenhouse
Effect which will cause the Earth to
lose its oceans. The Runaway Greenhouse Effect
will likely occur
in around 2 billion years.
|
 |
Mars: On Mars, a different path was followed. Mars initially had liquid
oceans and a mild, Earth-like climate. However, Mars then lost its
atmosphere rather quickly, perhaps over a few hundred million years but
certainly within a billion years of its formation. There is evidence that
the atmosphere of Mars has not changed for the last 3.7 to 3.8 billion
years based on the recent Mars missions.
This loss of its atmosphere led to the freezing of its oceans and to the
nearly atmosphereless, inhospitable state of the current Mars.
There were several thoughts about
what drove the atmospheric evolution on Mars:
(1) lack of plate tectonics;
(2) photodissociation by Solar ultraviolet radiation;
(3) large impacts on the surface of Mars; and
(4) interactions with the Solar Wind through, for example,
sputtering. The Mars
Atmosphere and Volatile Evolution Mission appears to have settled the
issue.
MAVEN: The Mars Atmosphere and Volatile Evolution mission studied the
interaction of the Solar Wind and the upper atmosphere of Mars. MAVEN
showed that the Solar Wind,
the stream of electrically charged
particles continuously blowing outward from the Sun
was a significant (if not dominant) contributor to the Mars's atmospheric
loss.
|
In the upper atmosphere,
molecules such as CO2 and
H2O are broken apart by Solar ultraviolet (UV)
radiation, the ions here are easy prey for the Solar Wind particles.
The Solar Wind particles strip them off (sputtering)
as they blow by at speeds of
400 km-s-1
(900,000 mph). The sputtering process is
efficient enough to have stripped as much as 80 to 90 %
of the CO2 from Mars since its birth.
This happened on Mars because, unlike Earth,
Mars lost its magnetic field early in its history and so lost the
ability to deflect the incoming Solar Wind. Rather, the Solar Wind's
charged particles crashed directly into the upper atmosphere of Mars
sputtering
off the carbon, hydrogen, and oxygen. Mars lost its
magnetic field after a few hundred million years,
because Mars is small in size and so develops
a cold interior rather quickly making it unable to generate
a magnetic field over its lifetime. |
 |
MAVEN has observed this process in action.
Below are pictures of carbon, oxygen, and hydrogen escaping from Mars.
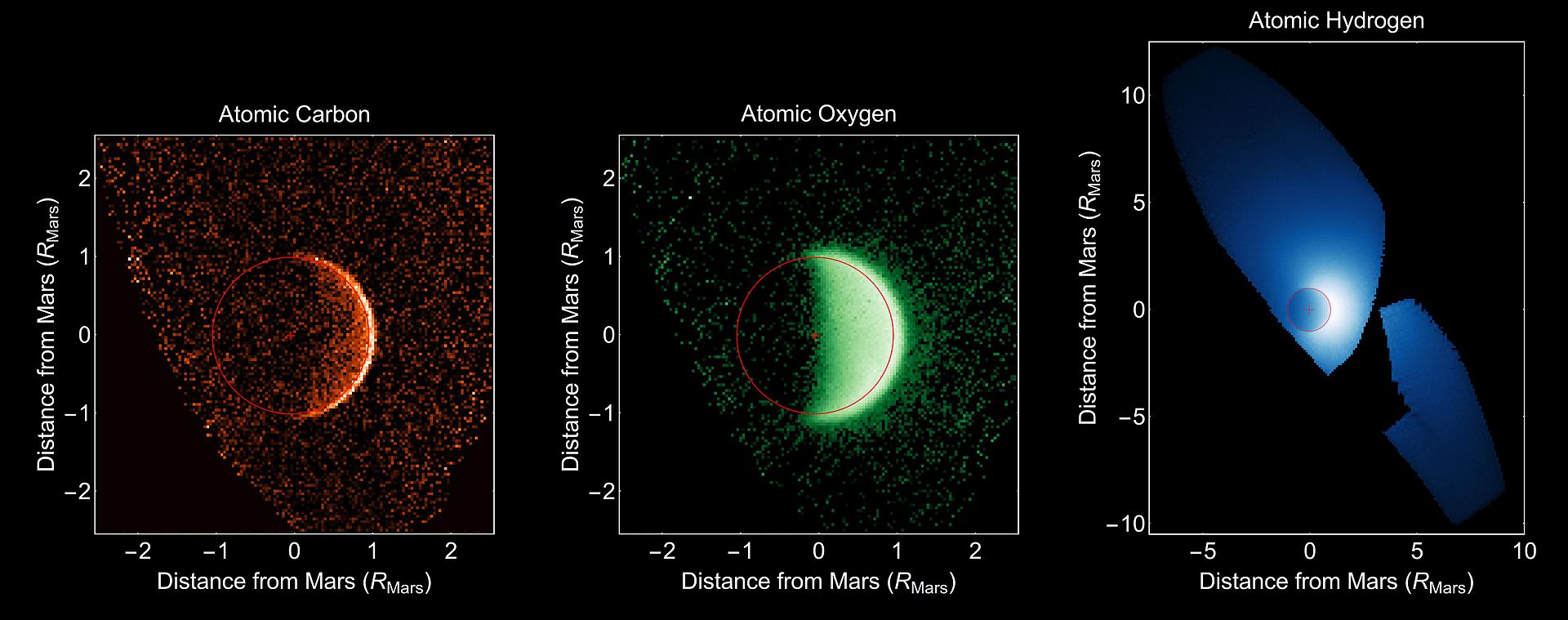 | |
|
|
Venus: On Venus, a third path was followed.
The evolution started similarly to the Earth and Mars. This is true even
though Venus is closer to the Sun than is the Earth and receives
twice the amount of Solar energy than does the Earth. With the higher
Solar energy input, the temperature on Venus rose high enough to prevent
the formation of ice, but it did not rise high enough
to prevent the condensation of atmospheric water vapor
into oceans. Shallow oceans formed. Venus was in this happy medium zone.
So, as on Earth and Mars, the oceans could start to
extract CO2 from the atmosphere and, as on Earth,
store some of ita sCO2 in its crust.
Unlike the Earth, however, as the
Sun aged and grew brighter,
Venus's oceans being a little warmer than on Earth started to
evaporate. This happens only because
Venus is slightly closer
to the Sun than is the Earth. The difference is not
large but it is enough.
Venus's oceans start to evaporate as the Sun brightens.
Again, what happens is that as the amount of water vapor
in Venus's atmosphere rises due to evaporation, the heating due to the
Greenhouse Effect increasexd, which leads to more evaporation and a stronger
Greenhouse Effect and so on. This positive feedback loop
again leads to a
Runaway Greenhouse effect
which vaporizes Venus's oceans. The water vapor then is slowly lost to
space as it rises high into the upper atmosphere where Solar UV radiation
breaks it apart and the hydrogen and oxygen atoms escape.
The CO2 does not escape but remains
in the atmosphere. The CO2
did not suffer the same fate as the CO2 on
Mars because of Venus's larger mass and stronger gravitational field.
The details of Venus's atmospheric evolution is a topic is current research
interest, a problem that is now of more than esoteric interest. Models for
the evolution of Venus's atmosphere are similar to those used for the
future evolution of the Terrestrial atmosphere.
 |
Data obtained by the Venus Express (ESA) studied the upper atmosphere of
Venus. Venus Express detected oxygen, hydrogen, and helium escaping from
Venus just as MAVEN had detected carbon, oxygen, and hydrogen escaping
from Mars. For Mars this meant CO2 and water H2O
were lost. For Venus, this meant that H2O and helium were lost
but that CO2 was not. Apparently because of Venus's
larger gravity, the heavier CO2 does not rise high enough in
the atmosphere to be broken down and lost through sputtering.
|
|
Summarize a few key points:
Earth:
The Earth maintains its mild climate (maintains its Greenhouse effect)
because a cycle was set up where volcanism returned carbon dioxide
to the atmosphere to balance the removal of carbon dioxide by the oceans,
plants, silicate weathering. The cycle has run
smoothly because of plate tectonics.
This happens because the Earth is
large enough to have a hot interior
which, by the way, also means that it has a magnetic field which shields us
from the Solar Wind. The final comment is that we are not as close to the
Sun as Venus, if we were, we would likely have also
suffered a Runaway Greenhouse Effect.
Mars:
Mars lost its
atmosphere in a few hundred million years due to sputtering by the Solar
Wind. This happened because Mars did not have a strong enough magnetic field
to deflect the Solar Wind particles. This situation likely arose
because Mars is small and so cannot maintain a hot interior for long times.
Venus:
On Venus, as the Sun aged and grew warmer
and brighter, Venus became hot enough for it to start to lose its
oceans through evaporation triggering the Runaway Greenhouse effect because
of the positive feedback loop described above. This
happened on Venus and not on Earth, because Venus
is a little closer to the Sun than is the Earth. The small difference
is enough to make Venus cross the threshold for evaporation early on
in its evolution, leading to the vaporization of its oceans.
|
Alternatively, Is it possible that Venus (and Mars)
never had water?
, so that we are answering a question that does not need to
be answered? This is not likely as
indicated by studies of the hydrogen isotope known as
deuterium (D).
Deuterium is a hydrogen atom whose nucleus contains a proton and
a neutron. A regular hydrogen atom's nucleus conains only a proton. Deuterium
is naturally occuring and on Earth we find that a certain fraction of water
molecules contains deuterium.
On Venus, the ratio D/H is around 120 times
larger than on Earth. This allows us to estimate the amount of water
Venus initially contained. Assume Venus loses its water as described
earlier. Deuterium, because it is more
massive than hydrogen, moves more slowly and thus less likely to escape
a planet's pull of gravity.
Calculations suggest that the current deuterium-to-hydrogen
(D/H) ratio on Venus means that Venus has
lost at least 99.9 % of its original water. Venus had water but lost it.
On Mars, the D/H ratio is around 5 times larger than on Earth,
which suggests that Mars has lost at least
75 % of its original water. Mars had water but lost it.
|
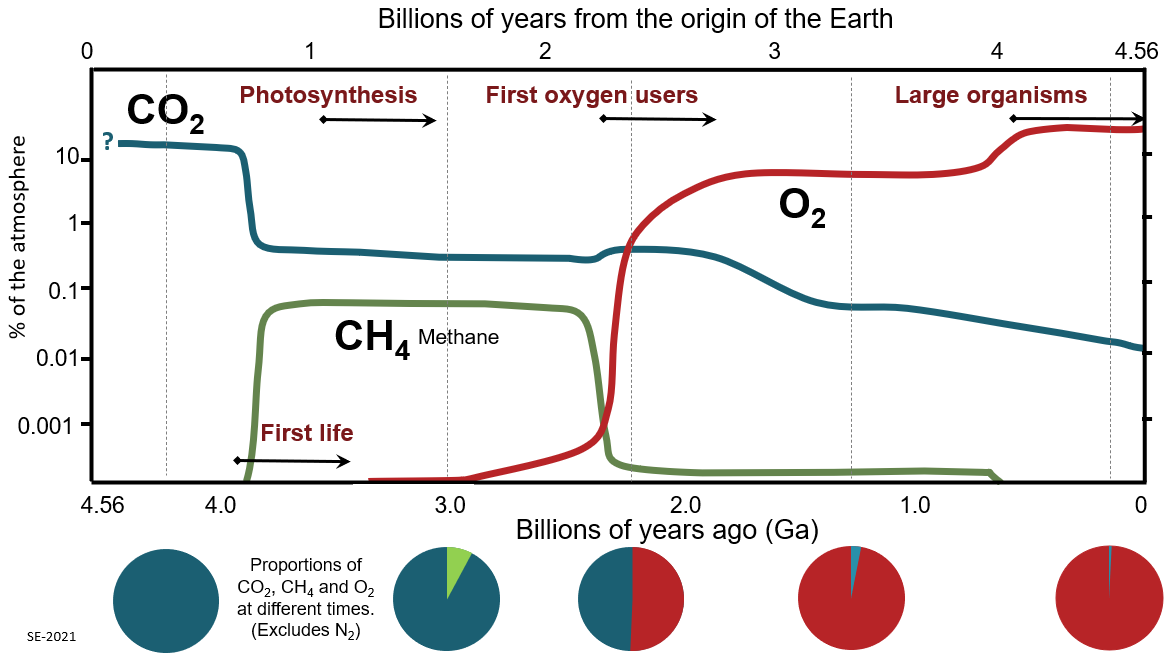
II. WHAT ABOUT THE OXYGEN IN THE EARTH'S ATMOSPHERE?
Today, Earth's atmosphere is
~21 % free oxygen. At birth, it had no
free oxygen. This is good, however, because chemical reactions
thought to produce amino acids are inhibited by oxygen.
Where did the oxygen come from?
(i) Photochemical dissociation -
breakup of water molecules by ultraviolet
produced free oxygen at ~ 1-2% levels. At these levels, ozone
can form to shield Earth surface from ultraviolet (UV) radiation.
(ii) Photosynthesis -
carbon dioxide + water + sunlight ===>
organic compounds + oxygen molecules. Produced by
cyanobacteria (photosynthetic prokareyotes--blue-algae), and
eventually higher plants supplied the rest of oxygen to the atmosphere.
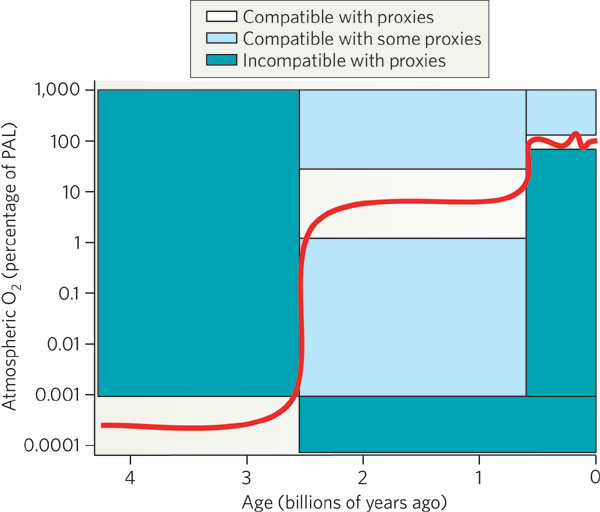
In the Archean period (4 billion years to 2.5 billion years ago),
there was little or no free oxygen in the atmosphere
(< 0.001 % of the current level of oxygen,
PAL). Around a billion years before the end of
the Archaen period, photosynthesis started.
The little oxygen
produced by cyanobacteria
was probably consumed by the weathering process. Only after
rocks at the surface were sufficiently
oxidized could free oxygen remain free in the atmosphere.
Interestingly, during the Archaen period, the day may have been as
short as 12.3 h with a year lasting 714 days. During this time, the
Moon slowed the Earth's rotation rate increasing the length of
the day. It was recently suggested that this may have led to
the jump in oxygen signaling the end of the Archean period. With a longer
day, cyanobacteria had a longer time for
uninterrupted photosynthesis which increased oxygen production.
During the Proterozoic era (2.5 to 0.5 billion years ago),
the free oxygen rose to 1 % to 40 % of PAL. Most
of the oxygen was released by cyanobacteria,
which showed a strong
increase in abundance (in the fossil record)
about 2.45 billion years ago. The present level of free oxygen
probably was achieved around ~400 million years ago which coincided with
a 3-fold increase in biodiversity on the Earth.
|
|










