![]()
Electricity |
 |
A) your mass is 100 times less on Jupiter.
B) Jupiter is significantly farther from the Sun.
C) Jupiter's radius is 10 times the Earth's radius.
D) Jupiter's radius is 100 times the Earth's radius.
E) you are 100 times more weightless there.
We call this motion (ball rolling of the end of table) projectile motion. It describes the motion of baseballs, cannonballs, divers at the pool, etc.
What do we notice about our projectile motion experiment?
- Both balls hit at the same time.
- One can time the drop of the one ball to determine how long the other ball moves horizontally.
- It is useful to know the time of flight of the other ball. If one knows its horizontal velocity, one can say how far (horizontally) it travels.
This information can be used to launch satellites!
- In launching satellites, we seek to match the curvature of the Earth.
- Satellites are groovy! (or bulgy?)
- Many orbits are "elliptical" instead of circles. What does that mean?
What is the energy situation of a satellite in orbit?
Circular orbit:
- Does its kinetic energy (KE) change as it orbits? Why or why not?
- Does its gravitational potential energy (GPE) change as it orbits? Why or why not?
- What about its total energy?
Elliptical orbit:
- Does its kinetic energy (KE) change as it orbits? Why or why not?
- Does its gravitational potential energy (GPE) change as it orbits? Why or why not?
- What about its total energy?
- What does the force look like on a satellite in an elliptical orbit?
![]()
Through various means we know that matter is comprised of atoms, and that atoms consist of protons, electrons and neutrons.
Charge is a property of electrons and protons, and serves to hold together atoms. Negatively-charged electrons can be mobile because they have 1/1830 of the mass of the positive proton. Neutrons have the same mass as a proton, but no charge. Also, protons and neutrons are found in the nuclei of atoms, and are held there by a different type of force.
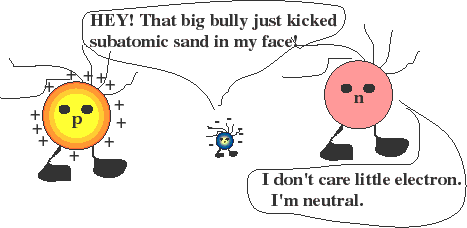
Protons are the original homebodies. They are NOT easily removed from a nucleus and do NOT constitute the positive charges that we see in physics class.
Instead, "holes" are the name physicists give to missing electrons. They are mobile like electrons and have effective positive charge.

Charge comes in bundles of specific size, as the proton's charge is the same as the electron's (but with opposite sign). The size of this bundle is called a quantum of charge. Charge comes in bundles that are indivisible.... one can have a charge of +1, +2, +3, etc, but not of 1/2 or 1/3. Charge is measured in Coulombs (C).
An object with -1 coulombs of charge has a net surplus of 6 x 1018 electrons! Clearly it is possible to separate many, many electrons from their "home atoms" and, thus create charged materials.
![]()
1) Forces between like charges: Take two pieces of scotch tape. Stick them to a table along most of their length. Rub them down so they adhere strongly to the table. Rip each piece off the table. You have just charged each piece in the same way with the same type of charge. Holding them by their ends, bring them near each other. What happens? Why?
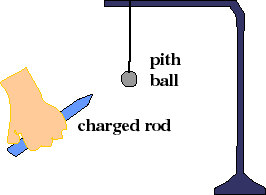
2) Forces between unlike charges: Charge a metal coated pith ball by rubbing a black rubber rod on cat fur. That makes the rod negative (excess electrons, remember its very negative to rub a kitty this much). Touching it to the pith ball allows some of the charges to escape to the pith ball. (Why?) Now charge a lucite (clear) rod by rubbing it on silk and hold it near the negatively-charged pith ball. What happens? The lucite rod is charged positively. What "rule" can you make after observing this phenomenon?
3) Forces between unlike charges: Take two different pieces of tape. Stick one to the table, then stick the other on the back of the first piece. Again, rub them so that they adhere well. Rip off the top piece of tape. Similar to before, its now charged. Where are the opposite, remaining charges? Rip off the second piece and hold the two pieces close together. What happens?
4) Polarization: Again charge the black rod negatively. Bring it near an uncharged pith ball. What happens first? What happens after the ball and rod touch? Can you describe the charge distribution on the pith ball before they touch? If so, why is the ball attracted to the rod?
5) Charging by induction: Use electrophorous plates as follows: Rub animal fur on the black (bakelite) surface of the device (its base). What is the net charge of this surface after doing so? Place the aluminum disk on the base. What happens to charges on the disk? Touch the aluminum disk. What happens next? What charge is left on the disk. Use the charged disk to light a gas discharge tube.
6) Charge polarization in water: Comb your hair with a black plastic comb. What is the charge on the comb afterwards? Place the charged comb near but not touching a small stream of water. What happens? Why?
Summary: The results of these simple experiments reinforce the ideas that like charges repel, unlike charges attract, and that the electric force between charge diminishes with distance.
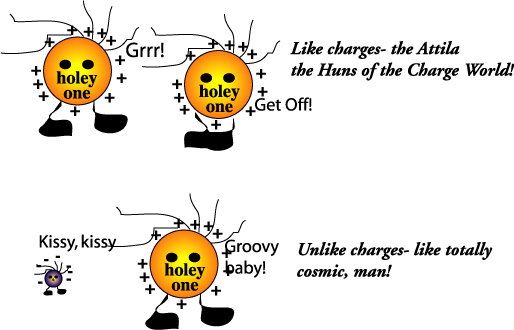
In saying that the charged rods/pith balls repel or attract, we really mean that they exert forces on one another. The negative charge on the metal surface of the pith ball is attracted to the positively-charged glass rod. It moves as close as it can get to it, bringing the pith ball with it. The oppositely charged pieces of tape are observed to move towards each other because of forces on their charges.
Try this experiment with the charged tape (either like or unlike charges). Hold them close enough together so that they just begin to deflect (either away from or towards one another). Now move them closer together. What happens? Why? Did the amount of excess charge on the tape change when you did this?
Experimentation shows that the force between two charged objects is proportional to the charge on each object and weakens according to the square of the distance separating the charged objects:
In this case, "q" is the standard symbol that means "amount of charge (in Coulombs, "C"). The separation is typically stated in meters (m). The constant for these units is:
For two objects with given charges q1 and q2, the force between them will diminish by 1/4 if the separation between the objects is increased by 2.
It turns out that this is exactly analogous to the force between two masses, which we have termed "the force of gravity." For gravity, however, we almost always are referring to the attraction of an object to the Earth. As the Earth is really quite large, moving small distances above its surface has very little affect on the force (and acceleration) of gravity.
To observe the electric force in action, we can watch what happens when styrofoam balls sitting on top of a Van de Graff generator accumulate excess charge from the generator:
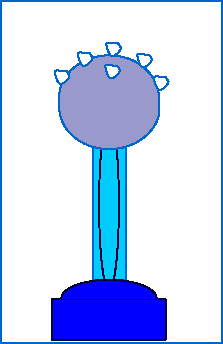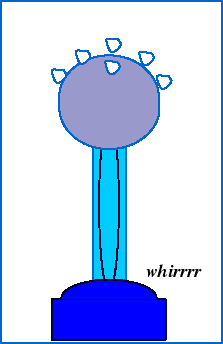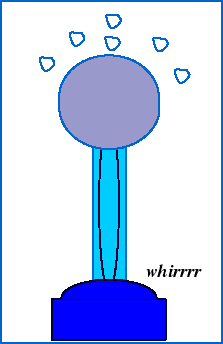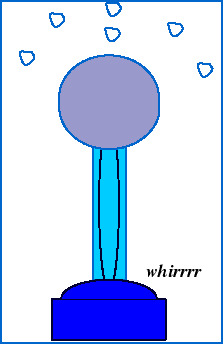
The shiny dome on top of the Van de Graaf generator appears to be made of a special substance. What is it? Why do you suppose it is made out of that?
Charge (electrons in this case) can move easily between metal atoms that are arranged in an orderly lattice. We call these substances conductors. Wires are conductors and are usually made of metal.
What about the sticky tape? Is it a good conductor of charge? How could you tell?
![]()