EVOLUTION OF THE ATMOSPHERES OF THE TERRESTRIAL PLANETS
|

|
We consider:
After this, we consider the atmospheres of Venus and Mars (and
address the question of why the atmospheres of the three planets
are so different).
I. ATMOSPHERE OF THE EARTH
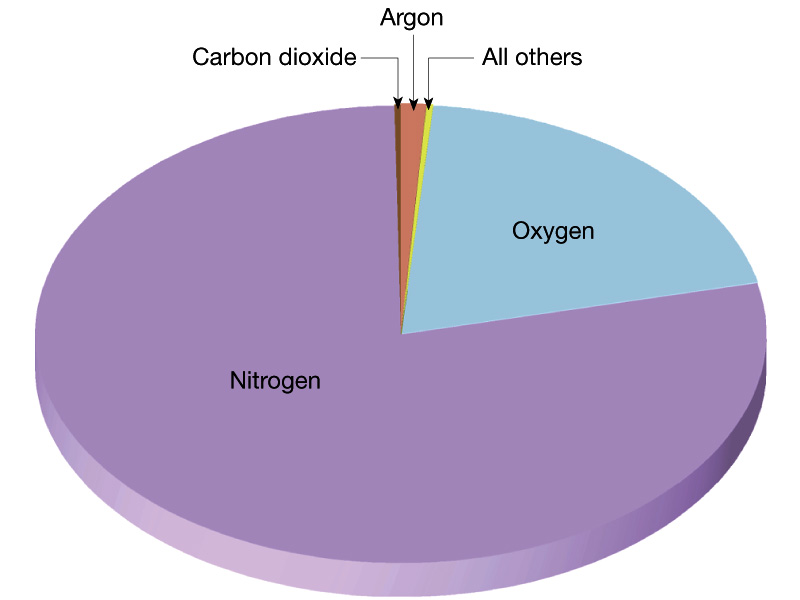
First look at the current atmosphere of the Earth. The current
atmosphere of the Earth has a pressure of 1 bar which is ~ 100 times
larger than Mars and ~ 1 % that of Venus. The
composition of the Earth's
atmosphere is 78 % Nitrogen molecules ad 21 % Oxygen
molecules with
trace amounts of other things, in particular, the greenhouse
gases water, carbon dioxide,
methane, and CFCs.
|
|
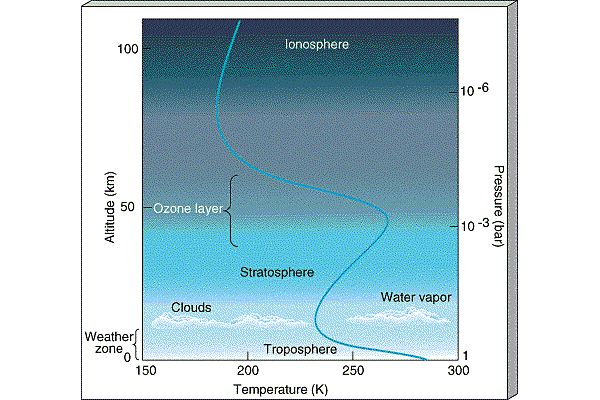
The atmosphere is conveniently divided into regions
in terms of how the temperature behaves (whether it is increasing
or decreasing):
|
- Thermosphere:
In the thermosphere, Solar radiation is
able to ionize
(strip electrons off of atoms forming the ionosphere
)
and temperature increases with altitude
(because atoms absorb Solar radiation).
The ionosphere is the layer
which traps radio signals and allows them to be heard around the
world (it is also the layer which gets disturbed and disrupts radio
communication during Solar storms).
- Mesosphere: There are no
strong absorbers of Solar radiation in the mesosphere so
temperature decreases with altitude there.
- Stratosphere:
The next layer of the atmosphere is known as the
Stratosphere and is broken up into layers
composed of different materials (i.e., it is
stratified from which follows its name). The stratosphere is the
layer where Ozone
lives. In the stratosphere, because Ozone
absorbs Solar ultraviolet radiation, temperature increases as
you move upward in altitude through the stratosphere.
- Troposphere:
The lowest layer of the atmosphere, the troposphere is
where atmospheric convection occurs and is the layer which
contains most of the water. The troposphere is the layer
where weather is generated. In the troposphere,
temperature declines with altitude. On average,
the temperature declines
with height at rate -6.5oC per kilometer in the lower
troposphere. At the top of the troposphere,
clouds form (because it gets too cold for water to be vapor).
This traps water in the troposphere, the so-called
Cold Trap. Because the ozone layer lies in the Stratosphere,
the water in the troposphere is shielded from the Solar UV radiation
and is not destroyed by photodissociaion.
|
II. ATMOSPHERIC EVOLUTION: EARTH, MARS, VENUS
Terrestrial planets (the atmosphere ones) are roughly
the same sizes and same distances from the Sun and yet, they have grossly
different kinds of atmospheres and conditions on their surfaces. Do we have
any ideas as to what leads to the huge differences? Surprisingly, there
may be simple explanations.
In the beginning, we believe that the material which was outgassed from the
interiors or carried in by comets onto the Terrestrial planets was
similar. That is, the Terrestrial planets started out roughly the same.
Originally, they were dominated by
water, carbon dioxide, sulfur dioxide, carbon monoxide, suflur, cholorine,
nitrogen, molecular hydrogen, sufur, nitrogen, and chlorine, ammonia, and
methane.
On each of Venus, Earth and Mars,
liquid oceans likely formed initially. On the Earth,
oceans formed in the Early Archean
period (the time before 2.5 billion years ago perhaps as long ago as
4 billion years). Despite all having started with
oceans only the Earth has retained extensive oceans.
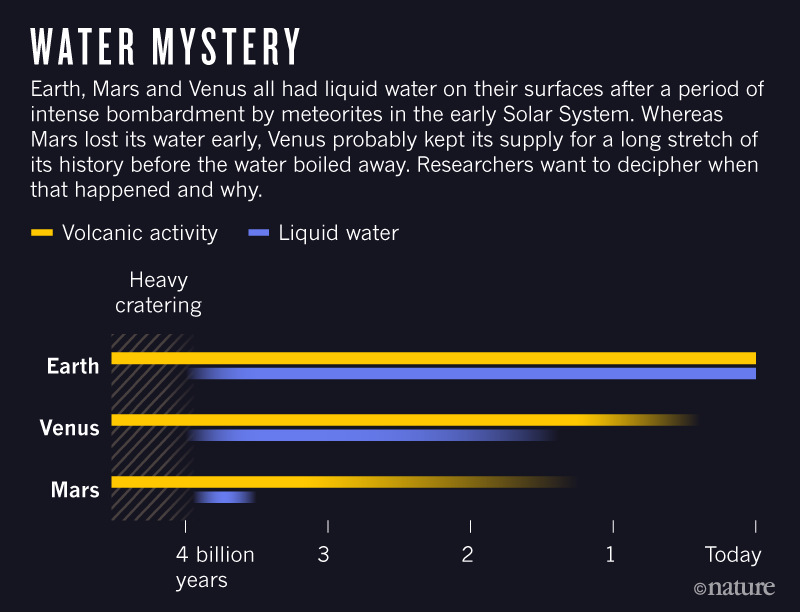
What caused the
difference in the evolution of the atmospheres of the Terrestrial planets
and liquid oceans as shown above?
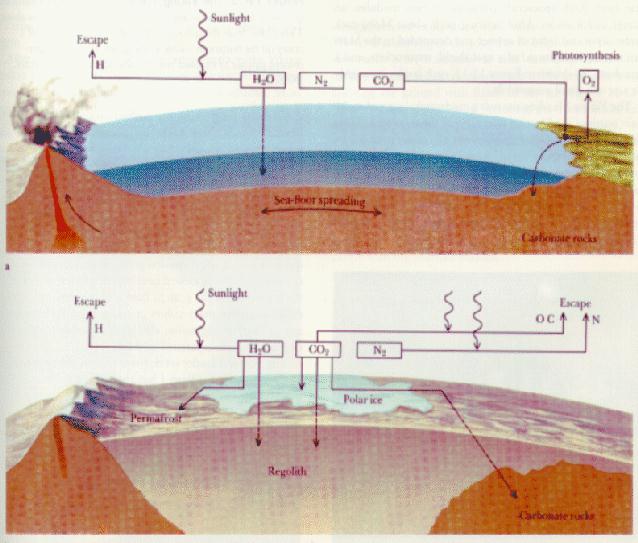
|
On Venus, Earth and Mars, carbon dioxide
initially dissolved into the oceans, was rained
out of the atmosphere
(and then washed into the oceans), or was directly adsorded into the
rocks and washed into the oceans. (The first two processes are less efficient
at higher temperatures.) Carbon dioxide
deposited into the oceans, settled
and formed sedimentary rocks ===> carbon dioxide was trapped
in the crust! This happened fairly quickly on Earth:

|
After this initial start-up, the evolutionary paths of Venus, Earth, and Mars
then diverged.
Earth: Recall that if the atmospheres of the early Terrestrial
planets had
no Greenhouse effect,
which would happen if they lost all of their carbon dioxide,
they would not have liquid oceans.
The Earth maintains its Greenhouse effect because
a cycle is set up where volcanism returns carbon dioxide
to the atmosphere. On the Earth, the cycle has run
fairly smoothly because of plate tectonics roughly
maintaining a carbon dioxide content that leads
to a temperate climate even though the Sun
becomes warmer and brighter as it ages.
What happens as the Sun continues to get brighter as
it ages? As the Sun's brightness increases, the biomass on the Earth will
increase removing CO2 from the atmosphere to give us a weaker
Greenhouse effect and keep the Earth temperate. This will work for a while but
it will not work after all of the CO2 is extracted from the
atmosphere. At this point, it is likely that the temperatures will rise and
the oceans will start to evaporate. This leads to more water
vapor and
so to a larger Greenhouse effect which leads to a higher temperature which
leads to more evaporation which leads to more water vapor and so on. This
positive feedback loop leads to a Runaway Greenhouse
Effect causing Earth
to lose its liquid oceans. This Runaway Greenhouse Effect
will likely occur
in around 2 billion years.
|
Mars: On Mars, a different path was followed. Mars initially had liquid
oceans and a mild, Earth-like climate. However, Mars lost its
atmosphere over the next few hundred million years.
This led to freezing of the oceans and its current inhospitable
state. It has been
determined that the atmosphere of Mars has not changed
greatly for the last 3.7-3.8 or so billion years,
so that the atmospheric evolution
was rather quick. It was sometime during the first billion years of Mars's
evolution that something caused it to lose the bulk of its
atmosphere leading to its rapid climate change.
There have been several thoughts about
what drove the atmospheric evolution on Mars:
(1) lack of plate tectonic activity;
(2) photodissociation by Solar ultraviolet radiation;
(3) large impacts on the surface of Mars; and
(4) interactions with the Solar Wind through, for example,
sputtering. Results from the Mars
Atmosphere and Volatile Evolution Mission appear to have settled the
issue:
MAVEN: Mars Atmosphere and Volatile Evolution mission results
show that the Solar Wind,
the stream of electrically charged
particles continuously blowing outward from the Sun,
was a significant (if not dominant) contributor to the atmospheric
loss.
|
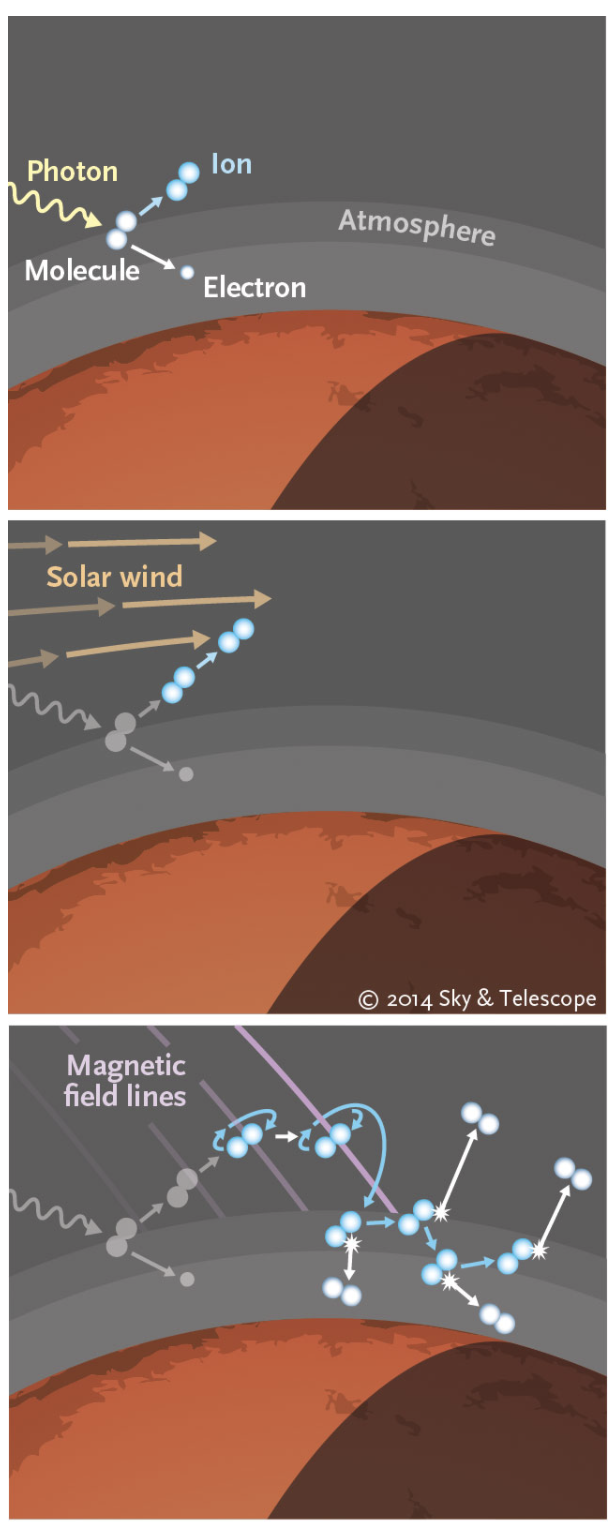
In the upper atmosphere where molecules such as
CO2 and
H2O have already been broken apart by Solar ultraviolet (UV)
radiation, the ions are easy prey for the Solar Wind particles
which strip them off (sputtering) as it blows by at
400 km-s-1
(900,000 mph). The process is efficient enough to have stripped
as much as 0.8 atmospheres of CO2 from Mars since its birth.
This happened because, unlike Earth,
Mars does not have a strong magnetic field to
deflect the incoming Solar Wind particles. Rather, the
charged particles crash directly into the upper atmosphere of Mars
sputtering off the carbon, hydrogen, and oxygen.
This happens because Mars has a cold interior and so is unable
to generate a strong magnetic field. |
 |
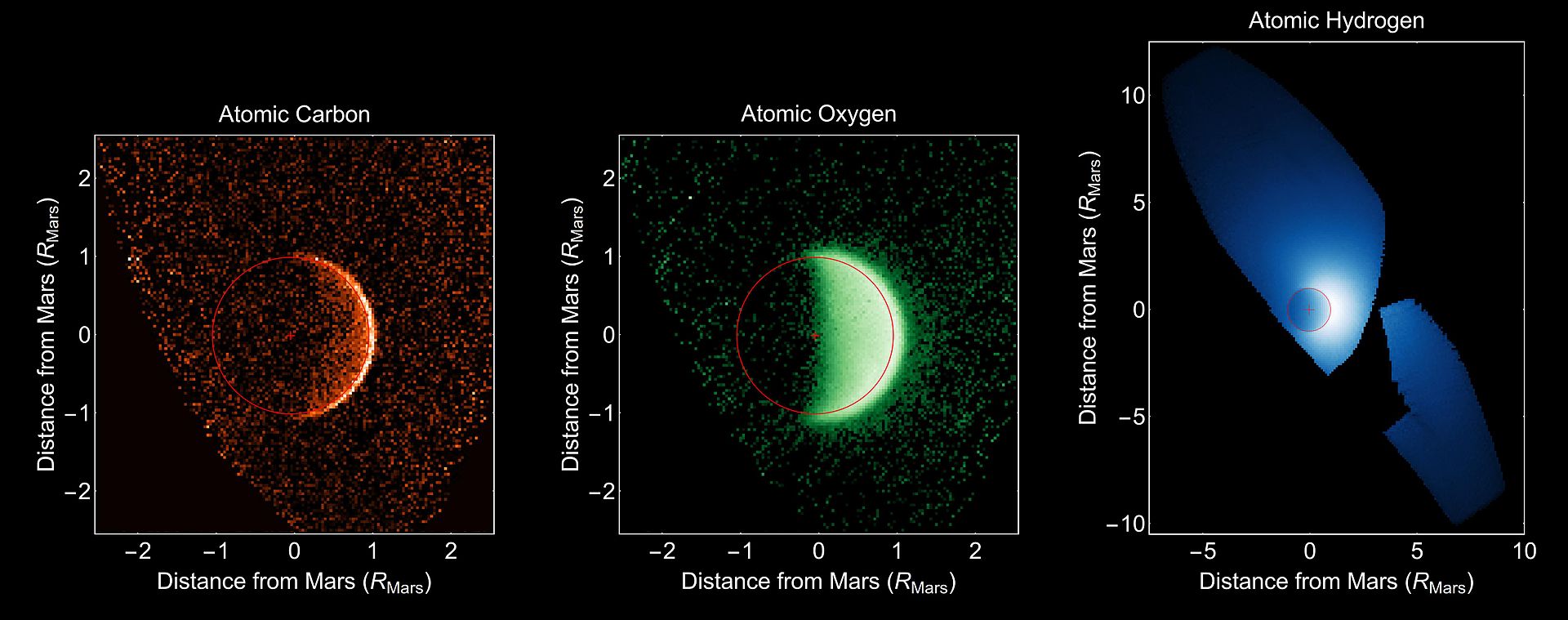
| MAVEN has observed this process in action bringing
scientists closer to solving the mystery of why the
ancient Martian climate is so different from the Martian climate of today.
(Below are pictures of carbon, oxygen, and hydrogen escaping from Mars.)
 |
|
|
Venus: On Venus, a third path was followed.
The evolution started similarly to the Earth and Mars. This is true
even though Venus is closer to the Sun than is the Earth and receives
twice the amount of Solar energy than does the Earth. Despite the higher
Solar energy input, the temperature on Venus was not high
enough to prevent the condensation of atmospheric water vaopr
into the oceans. The oceans could then
extract CO2 from the atmosphere and, as on Earth,
store the CO2 in its crust.
However, on Venus, as the Sun aged and grew warmer
and brighter, Venus started to
lose its oceans through evaporation unlike on the Earth, because Venus
was a little bit closer to the Sun than was the Earth. The small change
was enough to allow Venus to cross the threshold for
evaporation.
As the amount of water vapor
in Venus's atmosphere rose due to evaporation, the heating due to the
Greenhouse Effect increased, which led to more evaporation and a stronger
Greenhouse Effect. This positive feedback loop
led to a
Runaway Greenhouse effect
which vaporized the oceans. The water vapor was slowly lost to
space as it rose into the upper atmosphere where Solar UV radiation
broke it apart and the hydrogen and oxygen atoms escaped.
The Greenhouse effect was maintained, however, as the CO2
was baked
out of Venus's crust returning it to the atmosphere as Venus heated.
The details of how this works is a topic is current research interest,
a problem that is now of more than esoteric interest.
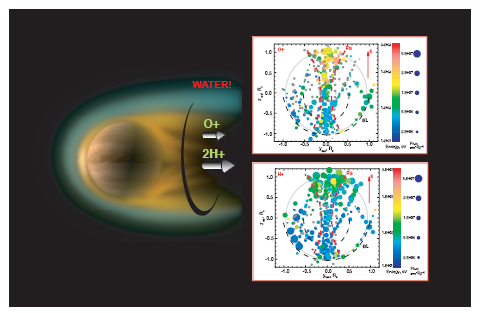 |
Data obtained by the Venus Express (ESA) studied the upper atmosphere of
Venus. Venus Express detected oxygen, hydrogen, and helium escaping from
Venus just as MAVEN had detected carbon, oxygen, and hydrogen escaping
from Mars. For Mars this meant CO2 and water H2O
were lost while for Venus, H2O and helium
but not CO2 were lost. Apparently because of Venus's
larger gravity, the heavier carbon dioxide does not rise high enough in the
atmosphere of Venus to be broken down and lost through sputtering.
|
Alternatively, Is it possible that Venus never had water?
This is not likely as
indicated by studies of the hydrogen isotope known as
deuterium (D).
Deuterium is a hydrogen atom whose nucleus contains a proton and
a neutron. A regular hydrogen atom's nucleus conains only a proton. Deuterium
is naturally occuring and on Earth we find that a certain fraction of water
molecules contains deuterium. On Venus, the ratio D/H is around 120 times
larger than on Earth. This allows us to estimate the amount of water
Venus initially contained. Assume Venus loses its water as described above.
Deuterium because it is more
massive than hydrogen moves more slowly, and thus less likely to escape.
Calculations suggest that the current deuterium-to-hydrogen
(D/H) ratio on Venus implies that Venus has
lost at least 99.9 % of its original water. |
|
Summarize a few key points:
Earth:
The Earth maintains its mild climate (maintains its Greenhouse effect)
because a cycle was set up where volcanism returned carbon dioxide
to the atmosphere to balance the removal of carbon dioxide by the oceans,
plants, ... . The cycle has run
smoothly because of plate tectonics.
This happens because the Earth is
large enough to have a hot interior
which, by the way, also means that it has a magnetic field which shields us
from the Solar Wind. The final good thing is that we are not as close to the
Sun as Venus, if we were, we would likely have also
suffered a Runaway Greenhouse Effect.
Mars:
Mars lost its
atmosphere in a few hundred million years due to sputtering by the Solar
Wind. This happened because Mars did not have a strong enough magnetic field
to deflect the Solar Wind particles. This situation likely arose
because Mars is small and so cannot maintain a hot interior for long times.
Venus:
On Venus, as the Sun aged and grew warmer
and brighter, Venus became hot enough for it to start to lose its
oceans through evaporation triggering a Runaway Greenhouse effect. This
happened on Venus and not on Earth, because Venus
is a little closer to the Sun than is the Earth. The small difference
is enough to make Venus cross the threshold for evaporation early on
in its evolution, leading to the vaporization of its oceans.
|
III. WHAT ABOUT THE FREE OXYGEN IN THE EARTH'S ATMOSPHERE?
Today, we see that the atmosphere of the Earth contains
~21 % free oxygen. As noted above, at birth there was no
free oxygen. This is good because chemical reactions thought to
produce amino acids are inhibited by oxygen
Where did the oxygen come from?
Oxygen Production:
(i) Photochemical dissociation -
breakup of water molecules by ultraviolet
produced free oxygen at ~ 1-2% levels. At these levels, ozone
can form to shield Earth surface from ultraviolet (UV) radiation.
(ii) Photosynthesis -
carbon dioxide + water + sunlight ===>
organic compounds + oxygen molecules. Produced by
cyanobacteria (photosynthetic prokareyotes--blue-algae), and
eventually higher plants supplied the rest of oxygen to the atmosphere.
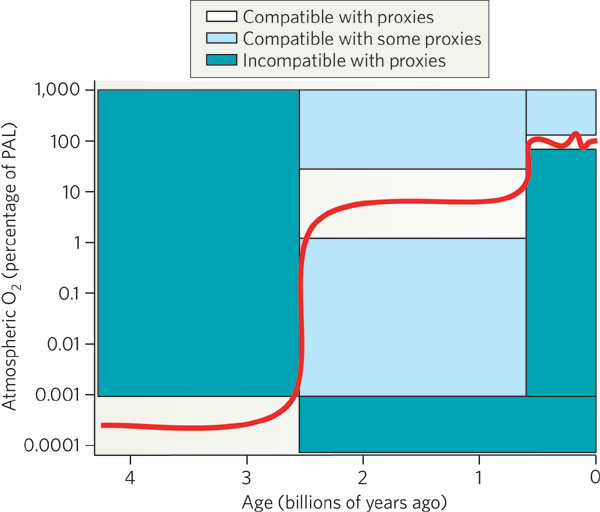
In the Archean period (4 billion years to 2.5 billion years ago),
there was little or no free oxygen in the atmosphere
(< 0.001 % of the current level of oxygen,
PAL). Around a billion years before the end of
the Archaen period, photosynthesis started.
The little oxygen
produced by cyanobacteria
was probably consumed by the weathering process. Only after
rocks at the surface were sufficiently
oxidized could free oxygen remain free in the atmosphere.
Interestingly, during the Archaen period, the day may have been as
short as 12.3 h with a year lasting 714 days. During this time, the
Moon slowed the Earth's rotation rate increasing the length of
the day. It was recently suggested that this may have led to
the jump in oxygen signaling the end of the Archean period. With a longer
day, cyanobacteria had a longer time for
uninterrupted photosynthesis which increased oxygen production.
During the Proterozoic era (2.5 to 0.5 billion years ago),
the free oxygen rose to 1 % to 40 % of PAL. Most
of the oxygen was released by cyanobacteria,
which showed a strong
increase in abundance (in the fossil record)
about 2.45 billion years ago. The present level of free oxygen
probably was achieved around ~400 million years ago which coincided with
a 3-fold increase in biodiversity on the Earth.
|
|











