Part 5D: EVOLUTION OF THE ATMOSPHERES OF THE TERRESTRIAL PLANETS |

|
Part 5D: EVOLUTION OF THE ATMOSPHERES OF THE TERRESTRIAL PLANETS |

|
|
 |
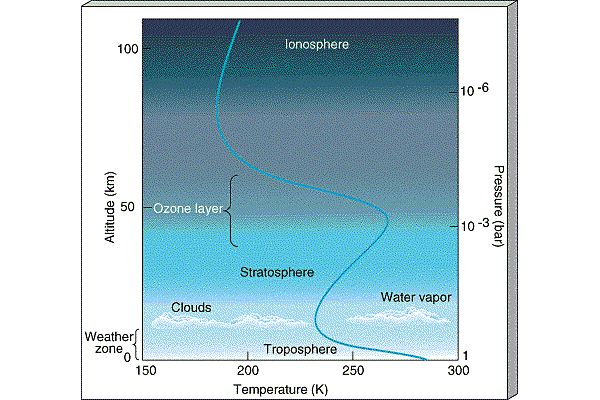
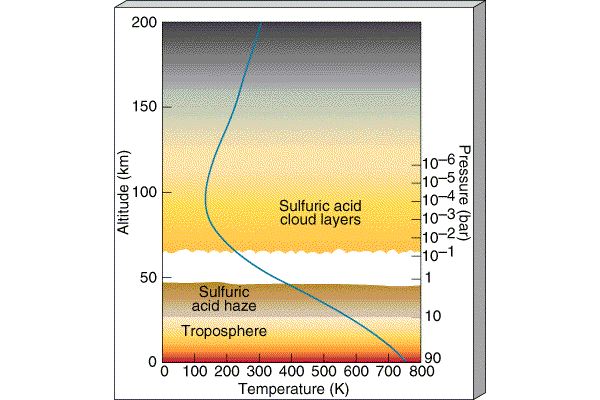
The atmosphere is conveniently divided into regions in terms of how the temperature behaves (whether it is increasing or decreasing):
|
|
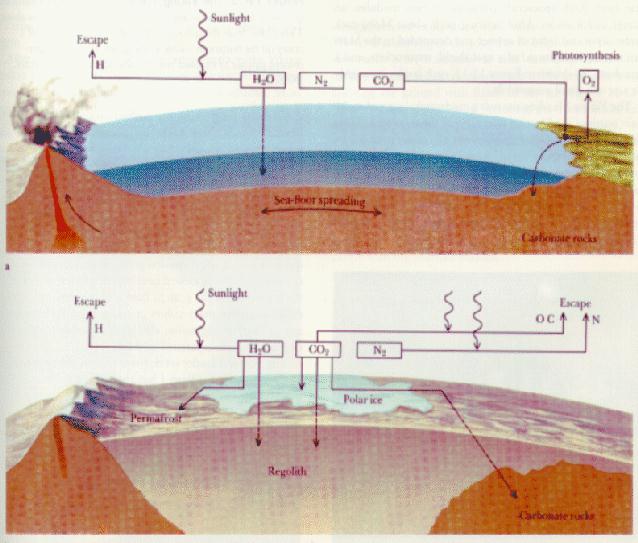
In the beginning, we believe that the material which was outgassed from the interiors or carried in by comets onto the Terrestrial planets was similar. That is, the Terrestrial planets started out roughly the same. Originally, they were dominated by water, carbon dioxide, sulfur dioxide, carbon monoxide, suflur, cholorine, nitrogen, molecular hydrogen, sufur, nitrogen, and chlorine, ammonia, and methane.As the Earth evolved and cooled liquid water was able to form. There is evidence that there was liquid water as long as 4.2 billion years ago and that it was never so hot that liquid water could not exist. Extensive oceans clearly formed by early in the Early Archean period (the time between 4 billion to 2.5 billion years ago) as the Earth cooled. What are the consequences of the formation of extensive liquid oceans?
On the Earth and Mars, water vapor likely dominated the early atmosphere, but because of the temperatures, liquid ocean formed:
|
On Earth and possibly
Mars, carbon dioxide combined with water
to form carbonic acid in the atmosphere which was rained
out of the atmosphere forming calcium, magnesium, potassium or
sodium ions on the urfae which were then washed into the oceans; or
the carbon dioxide was directly adsorded into the
rocks and washed into the oceans. Carbon dioxide
deposited into the oceans, settled
and formed sedimentary rocks ===> carbon dioxide was trapped
in the crust! This happens fairly quickly: |
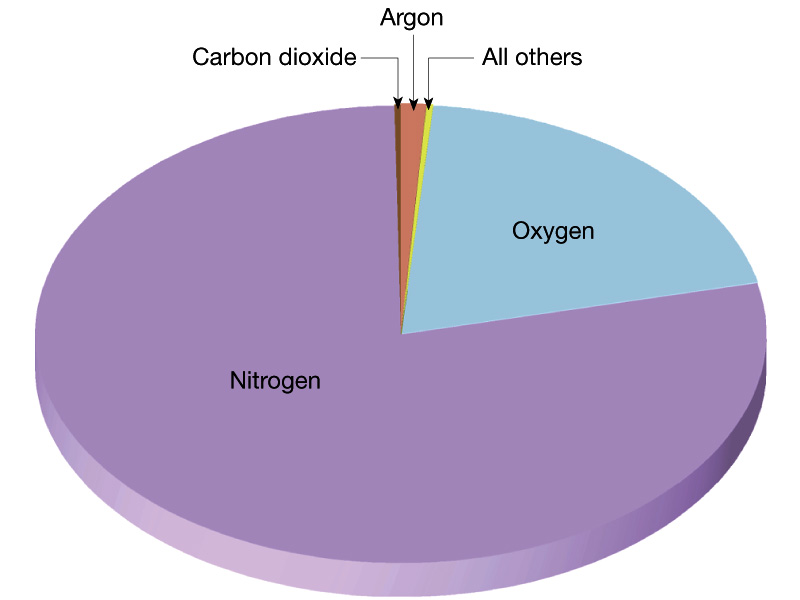
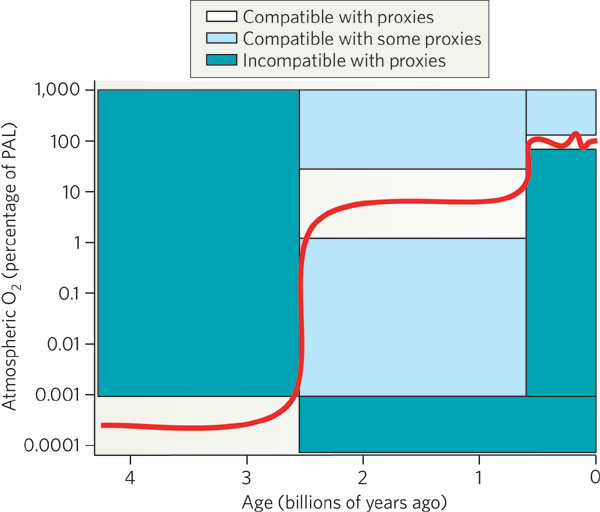
What About the Free Oxygen in the Earth's Atmosphere?Today, we see that the atmosphere of the Earth contains ~21 % free oxygen. As noted above, at birth there was no free oxygen. This is good because chemical reactions thought to produce amino acids are inhibited by oxygen Where did the oxygen come from? Oxygen Production:
(ii) Photosynthesis - carbon dioxide + water + sunlight ===> organic compounds + oxygen molecules. Produced by cyanobacteria (photosynthetic prokareyotes--blue-algae), and eventually higher plants supplied the rest of oxygen to the atmosphere. During the Proterozoic era (1.8 to 0.5 billion years ago), the free oxygen rose slightly to 1 % to 40 % of PAL as the rocks became less efficient at taking up free oxygen. Most of the oxygen was released by cyanobacteria, as evidenced by an increase in abundance (in the fossil record) about 2.45 to 1.8 billion years ago. Around 400 million years ago, the crust was no filled with oxygen and could no longer take up the released free oxygen; the present level of free oxygen probably was achieved around this time, ~400 million years ago. |
|
Evidence from the Rock Record:
(i) iron is extremely reactive with oxygen. If we look at the
oxidation state of Fe in the rock record, we can infer much
about atmospheric evolution.
In the Archean period, we find
minerals in sediments that can only form in non-oxidizing
environments: Pyrite (Fool's gold), and Uraninite.
These minerals are easily dissolved out of rocks under present
atmospheric conditions.
Banded Iron Formation (BIF) - Deep water deposits in which layers of
iron-rich minerals alternate with iron-poor layers, primarily chert.
Iron minerals include iron oxide, iron carbonate, iron silicate, iron
sulfide. BIF's are a major source of iron ore,
because they contain magnetite
which has a higher iron-to-oxygen ratio than hematite. These are
common in rocks 2.0 - 2.8 billion years old, but do not form today.
Red beds (continental siliciclastic deposits) are never found in rocks
older than 2.3 Billion years, but are common during the Phanerozoic time.
Red beds are red because of the highly oxidized mineral hematite
that probably forms by oxidation of other Fe minerals that have
accumulated in the sediment.
Conclusion - amount of free oxygen in the atmosphere has increased with time.
Biological Evidence:
Chemical building blocks of life could not have formed in the presence of
free oxygen. Chemical reactions that yield amino acids
are inhibited by the presence of even very small amounts of oxygen.
Oxygen also prevents the growth of many primitive bacteria such as
photosynthetic bacteria, methane-producing bacteria and bacteria that derive
energy from fermentation.
Conclusion - Today, most primitive life forms are
anaerobic suggesting that the first forms of cellular life
probably also had similar metabolism.
Today, such anaerobic life forms are restricted to anoxic (low oxygen)
habitats such as swamps, ponds, and lagoons.
Atmospheric oxygen built up in the early history of the Earth as the waste
product of photosynthetic organisms and by burial of organic matter away
from surficial decay. This history is documented by the geologic
preservation of oxygen-sensitive minerals,
deposition banded iron formations, and development of continental
"red beds" or BIFs.
Because there is no Ozone layer,
the temperature simply decreases as you move up in
altitude around Venus. There is not a water trap and the
water vapor is free to rise up
into the high levels of Venus's
atmosphere where it is broken up by Solar radiation. The hydrogen atoms from
the water then escape to space and the oxygens combine with other atmospheric
gases to form different molecules. Venus thus loses its water. After the
water is lost, the Greenhouse effect eases and the temperature drops to
the mild ~ 800-900 F of today and the pressure drops to 90 bars.
An upshot of the above scenario is that in the past Mars could have had a much
thicker atmosphere and been much more earth-like (there are models which
suggest that the early Mars had an atmospheric pressure of 2 bars!). This
is interesting because, today,
the atmospheric conditions on Mars are such that liquid water cannot
exist on the surface of Mars.
We do see evidence, however, for water on Mars. For example,
there is
water in the northern residual polar ice cap:
In addition to the water in the northern residual polar caps, there is
also evidence for water in the low-lying clouds above canyons, and in
large glaciers lying scattered rocky debris:
There, presumably, is also a permafrost layer on Mars even today as
implied by
Outflow Channels (large channels which can be up to 100 kilometers and
thousands of kilometers long--likely formed by catastrophic flooding),
"Islands", and
Splosh Craters (oozing mud formed by impacts which melted
the permafrost layer).
The outflow channels and islands were produced by massive floods on
Mars. Presumably what happened was that some event (possibly the impact
of a large object) caused a
rapid, large-scale melting of the permafrost layer which caused floods.
These is ample evidence that water exists on Mars much of it
below the surface which can be
melted and lead to transient flows.
There is also evidence that in the past
water existed in liquid form
on the surface of Mars under quiescent conditions
===> grossly different atmospheric
conditions in the past than presently.
There is thus
evidence that the climate of Mars may have been more Earth-like in the
past than it is today. This leads to the hope that perhaps
life existed
on Mars in the past.
Life on Mars?
Where is Venus's Water?
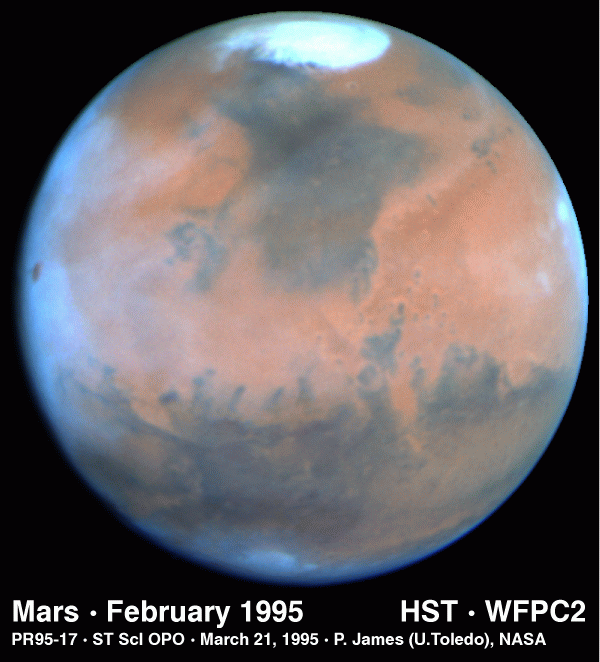
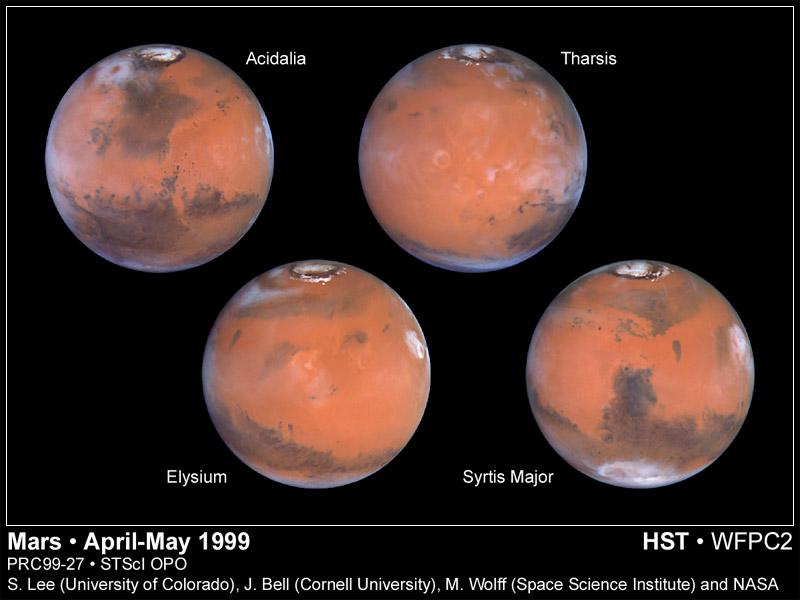
The polar caps on Mars have two parts; regions that show
seasonal variations and
residual caps. The seasonal caps are thought to be composed of frozen
carbon dioxide. The residual caps are smaller and brighter than the
seasonal caps and show a very marked north-south asymmetry. The southern
residual cap is frozen carbon dioxide while it is believed that the
northern residual cap is water ice (supported by the observation that water
vapor is observed over the residual cap in the northern summer and the
temperatures of the caps).
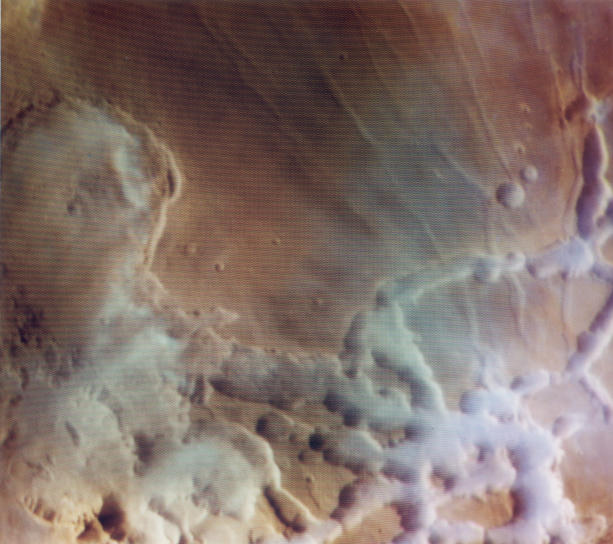

Huge glaciers up to half a mile thick which lie close to the equator of Mars
are thought to be the remnants of an ice age on Mars.
It is thought that the glaciers formed up to 100 million
years ago and represent evidence of climate change on Mars.
Hundreds of glaciers have been identified by researchers using
ground-penetrating radar which allows them to see through the
rocky layers of debris covering the ice. The largest glacier
is 13 miles long and more than 60 miles wide. It could be a
source of water for astronauts on Mars. When the glaciers
formed, Mars' climate was much colder because the angle Mars' spin axis
makes with its orbitl axis was much greater than it is now. This allowed
ice sheets to extend far beyond the polar regions and towards,
possibly even reaching, the Equator.
![]()


