| Readings: |
Dalton States of Matter Thermodynamics Rutherford atom |
Atomic Theory:

The ancient philosopher, Heraclitus, maintained that everything is in a state of flux. Nothing escapes change of some sort (it is impossible to step into the same river). On the other hand, Parmenides argued that everything is what it is, so that it cannot become what is not (change is impossible because a substance would have to transition through nothing to become something else, which is a logical contradiction). Thus, change is incompatible with being so that only the permanent aspects of the Universe could be considered real.
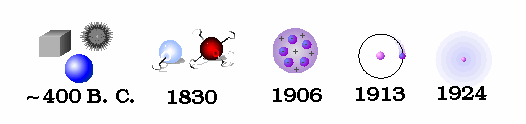
An ingenious escape was proposed in the fifth century B.C. by Democritus. He hypothesized that all matter is composed of tiny indestructible units, called atoms. The atoms themselves remain unchanged, but move about in space to combine in various ways to form all macroscopic objects. Early atomic theory stated that the characteristics of an object are determined by the shape of its atoms. So, for example, sweet things are made of smooth atoms, bitter things are made of sharp atoms.
In this manner permanence and flux are reconciled and the field of atomic physics was born. Although Democritus' ideas were to solve a philosophical dilemma, the fact that there is some underlying, elemental substance to the Universe is a primary driver in modern physics, the search for the ultimate subatomic particle.
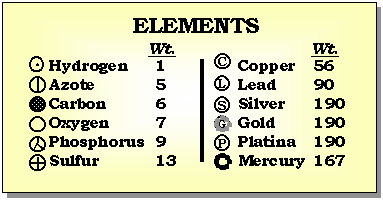
It was John Dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. In addition, he formulated the theory that a chemical combination of different elements occurs in simple numerical ratios by weight, which led to the development of the laws of definite and multiple proportions.
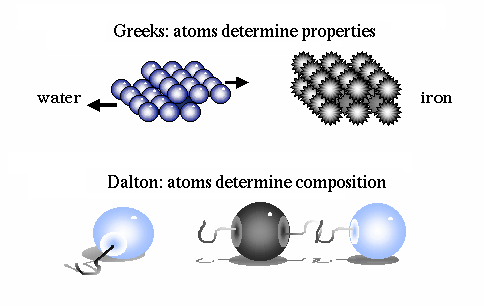
He then determined that compounds are made of molecules, and that molecules are composed of atoms in definite proportions. Thus, atoms determine the composition of matter, and compounds can be broken down into their individual elements.
The first estimates for the sizes of atoms and the number of atoms per unit volume where made by Joesph Loschmidt in 1865. Using the ideas of kinetic theory, the idea that the properties of a gas are due to the motion of the atoms that compose it, Loschmidt calculated the mean free path of an atom based on diffusion rates. His result was that there are 6.022x1023 atoms per 12 grams of carbon. And that the typical diameter of an atom is 10-8 centimeters.
By the 19th century, it was determined that atoms bind together to form substances through electromagnetic forces. That atoms are very small and matter is mostly empty space, but 'feels' solid because the atoms of your hand are repelled by the electromagnetic forces between your atoms and an objects atoms (like a table).
Matter:
Matter exists in four states: solid, liquid, gas and plasma. Plasmas are only found in the coronae and cores of stars. The state of matter is determined by the strength of the bonds between the atoms that makes up matter. Thus, is proportional to the temperature or the amount of energy contained by the matter.
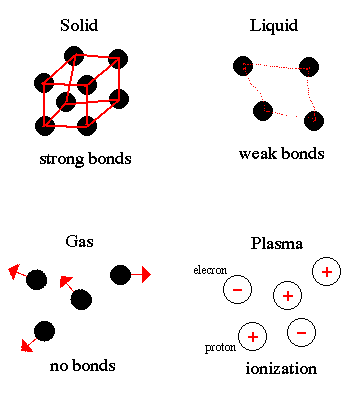
The change from one state of matter to another is called a phase transition. For example, ice (solid water) converts (melts) into liquid water as energy is added. Continue adding energy and the water boils to steam (gaseous water) then, at several million degrees, breaks down into its component atoms.
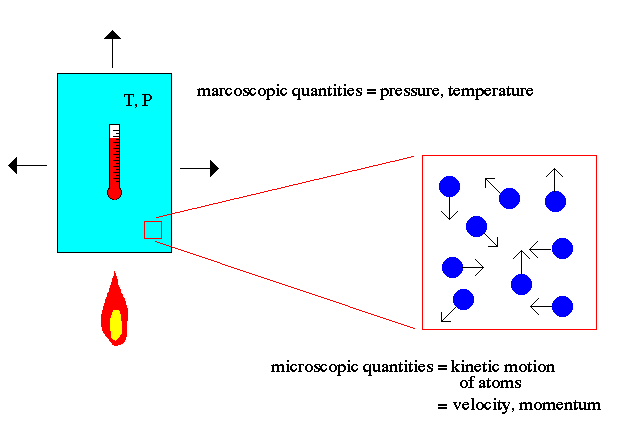
The key point to note about atomic theory is the relationship between the macroscopic world (us) and the microscopic world of atoms. For example, the macroscopic world deals with concepts such as temperature and pressure to describe matter. The microscopic world of atomic theory deals with the kinetic motion of atoms to explain macroscopic quantities.
Temperature is explained in atomic theory as the motion of the atoms (faster = hotter). Pressure is explained as the momentum transfer of those moving atoms on the walls of the container (faster atoms = higher temperature = more momentum/hits = higher pressure).
Ideal Gas Law:
Macroscopic properties of matter are governed by the Ideal Gas Law of chemistry.
An ideal gas is a gas that conforms, in physical behavior, to a particular, idealized relation between pressure, volume, and temperature. The ideal gas law states that for a specified quantity of gas, the product of the volume (V), and pressure (P), is proportional to the absolute temperature (T) and the number or density of particles (n),; i.e., in equation form, PV = nkT, in which k is a constant (called Boltzmann's constant). Such a relation for a substance is called its equation of state and is sufficient to describe its gross behavior.
Although no gas is perfectly described by the above law, the behavior of real gases is described quite closely by the ideal gas law at sufficiently high temperatures and low pressures (such as air pressure at sea level), when relatively large distances between molecules and their high speeds overcome any interaction. A gas does not obey the equation when conditions are such that the gas, or any of the component gases in a mixture, is near its triple point.
The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random motion and obey Newton's deterministic laws of motion; (2) the volume of the molecules is negligibly small compared to the volume occupied by the gas; and (3) no forces act on the molecules except during elastic collisions of negligible duration.
Thermodynamics:
The study of the relationship between heat, work, temperature, and energy, encompassing the general behavior of physical system is called thermodynamics. There are four laws to thermodynamics, all dealing with energy and its evolution from system to system.
The zeroth law of thermodynamics is that two systems are in thermal equilibrium with a third system, they must be in thermal equilibrium with each other. This law helps define the notion of temperature, and places the known fact that all systems attempt to reach equilibrium. States or systems not in equilibrium are forced and require an explanation, such as lifeforms.
The first law of thermodynamics is often called the law of the conservation of energy (actually mass-energy) because it says, in effect, that when a system undergoes a process, the sum of all the energy transferred across the system boundary--either as heat or as work--is equal to the net change in the energy of the system. For example, if you perform physical work on a system (e.g. stir some water), some of the energy goes into motion, the rest goes into raising the temperature of the system.
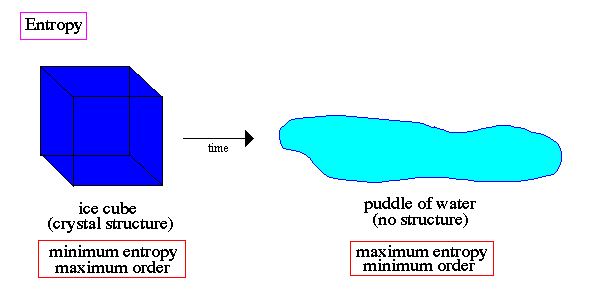
Classical or Newtonian physics is incomplete because it does not include irreversible processes associated with the increase of entropy. The entropy of the whole Universe always increased with time. We are simply a local spot of low entropy and our destiny is linked to the unstoppable increase of disorder in our world => stars will burn out, civilizations will die from lack of power.
The approach to equilibrium is therefore an irreversible process. The tendency toward equilibrium is so fundamental to physics that the second law is probably the most universal regulator of natural activity known to science.
The concept of temperature enters into thermodynamics as a precise mathematical quantity that relates heat to entropy. The interplay of these three quantities is further constrained by the third law of thermodynamics, which deals with the absolute zero of temperature and its theoretical unattainability.
Absolute zero (approximately -273 C) would correspond to a condition in which a system had achieved its lowest energy state. The third law states that, as this minimum temperature is approached, the further extraction of energy becomes more and more difficult.
Rutherford Atom:
Ernest Rutherford is considered the father of nuclear physics. Indeed, it could be said that Rutherford invented the very language to describe the theoretical concepts of the atom and the phenomenon of radioactivity. Particles named and characterized by him include the alpha particle, beta particle and proton. Rutherford overturned Thomson's atom model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny, massive nucleus.

His results can best explained by a model for the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun.
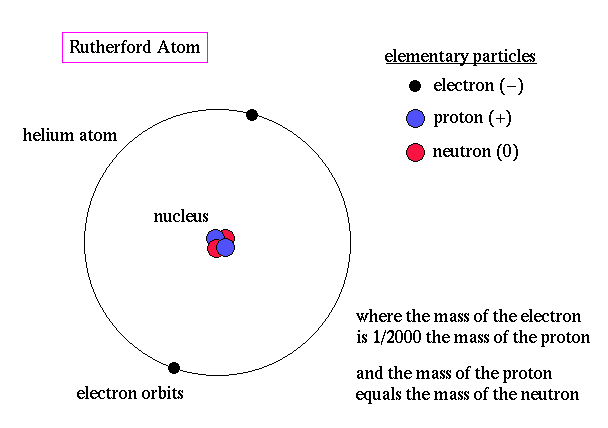
The Rutherford atomic model has been alternatively called the nuclear atom, or the planetary model of the atom.

|
|

|