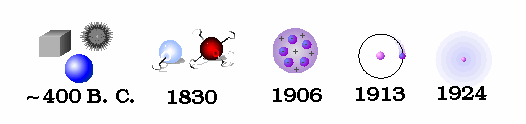

Atomic Theory:
The ancient philosopher, Heraclitus, maintained that everything is in a state of flux. Nothing escapes change of some sort (it is impossible to step into the same river). On the other hand, Parmenides argued that everything is what it is, so that it cannot become what is not (change is impossible because a substance would have to transition through nothing to become something else, which is a logical contradiction). Thus, change is incompatible with being so that only the permanent aspects of the Universe could be considered real.
An ingenious escape was proposed in the fifth century B.C. by Democritus. He hypothesized that all matter (plus space and time) is composed of tiny indestructible units, called atoms. This idea seems motivated by the question of how finely one can go on cutting up matter. While Democritus performed no experiments and had only the flimsiest evidence for postulating the existence of atoms, his theory was kept alive by the Roman poet Lucretius which survived the Dark Ages to be discovered in 1417. The atoms in Democritus theory themselves remain unchanged, but move about in space to combine in various ways to form all macroscopic objects. Early atomic theory stated that the characteristics of an object are determined by the shape of its atoms. So, for example, sweet things are made of smooth atoms, bitter things are made of sharp atoms.
In this manner permanence and flux are reconciled and the field of atomic physics was born. Although Democritus' ideas were to solve a philosophical dilemma, the fact that there is some underlying, elemental substance to the Universe is a primary driver in modern physics, the search for the ultimate subatomic particle.
It was John Dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. In addition, he formulated the theory that a chemical combination of different elements occurs in simple numerical ratios by weight, which led to the development of the laws of definite and multiple proportions.
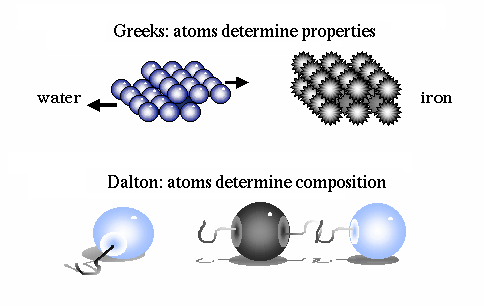
He then determined that compounds are made of molecules, and that molecules are composed of atoms in definite proportions. Thus, atoms determine the composition of matter, and compounds can be broken down into their individual elements.
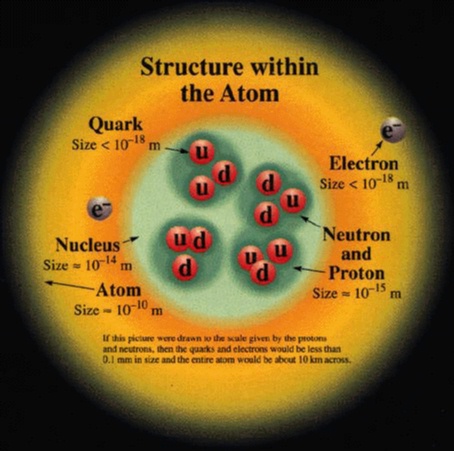
The first estimates for the sizes of atoms and the number of atoms per unit volume where made by Joesph Loschmidt in 1865. Using the ideas of kinetic theory, the idea that the properties of a gas are due to the motion of the atoms that compose it, Loschmidt calculated the mean free path of an atom based on diffusion rates. His result was that there are 6.022x1023 atoms per 12 grams of carbon. And that the typical diameter of an atom is 10-8 centimeters.
Matter:
Matter exists in four states: solid, liquid, gas and plasma. Plasmas are only found in the coronae and cores of stars. The state of matter is determined by the strength of the bonds between the atoms that makes up matter. Thus, is proportional to the temperature or the amount of energy contained by the matter.
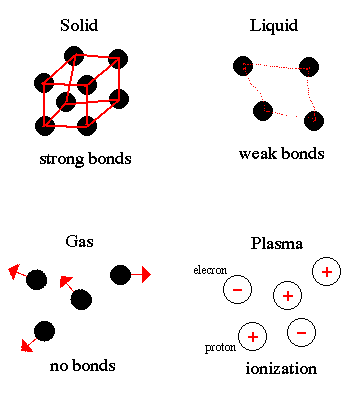
The change from one state of matter to another is called a phase transition. For example, ice (solid water) converts (melts) into liquid water as energy is added. Continue adding energy and the water boils to steam (gaseous water) then, at several million degrees, breaks down into its component atoms.
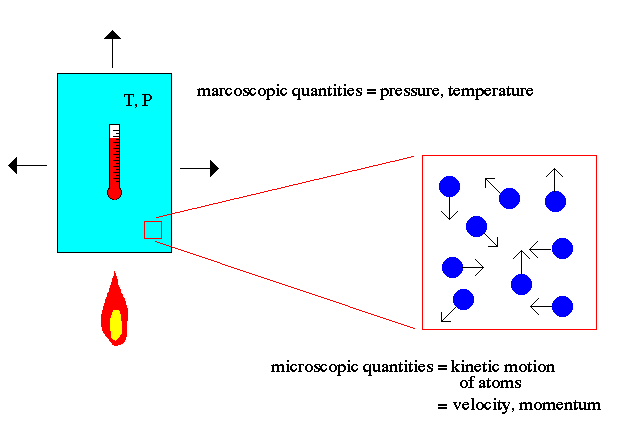
Atomic theory is the field of physics that describes the characteristics and properties of atoms that make up matter. The key point to note about atomic theory is the relationship between the macroscopic world (us) and the microscopic world of atoms. For example, the macroscopic world deals with concepts such as temperature and pressure to describe matter. The microscopic world of atomic theory deals with the kinetic motion of atoms to explain macroscopic quantities.
Temperature is explained in atomic theory as the motion of the atoms (faster = hotter). Pressure is explained as the momentum transfer of those moving atoms on the walls of the container (faster atoms = higher temperature = more momentum/hits = higher pressure).
Thomson Atom:
The next great step forward in the understanding of atoms was accomplished by John Thomson. Using a cathode ray scope, Thomson determined that all matter, whatever its source, contains particles of the same kind that are much less massive than the atoms of which they form a part. They are now called electrons, although he originally called them corpuscles.

His discovery was the result of an attempt to solve a long-standing controversy regarding the nature of cathode rays, which occur when an electric current is driven through a vessel from which most of the air or other gas has been pumped out.
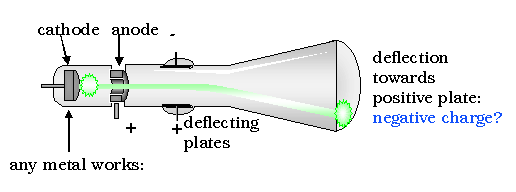
By applying an improved vacuum technique, Thomson was able to put forward a convincing argument that these rays were composed of particles. Furthermore, these rays seemed to be composed of the same particles, or corpuscles, regardless of what kind of gas carried the electric discharge or what kinds of metals were used as conductors. From these ideas he developed the idea that atoms are made of negative electrons embedded in a gel of positive charge (a "plum pudding" model).
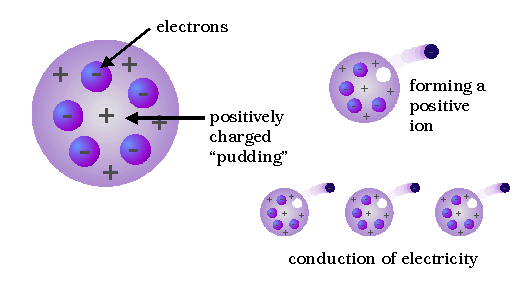
Thomson's conclusion that the corpuscles were present in all kinds of matter was strengthened during the next three years, when he found that corpuscles with the same properties could be produced in other ways; e.g., from hot metals. Thomson may be described as "the man who split the atom" for the first time, although "chipped" might be a better word, in view of the size and number of electrons.
Rutherford Atom:
Ernest Rutherford is considered the father of nuclear physics. Indeed, it could be said that Rutherford invented the very language to describe the theoretical concepts of the atom and the phenomenon of radioactivity. Particles named and characterized by him include the alpha particle, beta particle and proton. Rutherford overturned Thomson's atom model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny, massive nucleus.

By the turn of the 20th century, physicists knew that certain elements emitted fast moving particles of two flavors, alpha particles and beta particles. These elements were typically very heavy (i.e. their atom nuclei were massive) such as uranium and radium. Today we know that heavy nuclei are unstable and `decay', meaning that they spontaneously split into smaller nuclei and emit stray particles. This is called radioactivity.
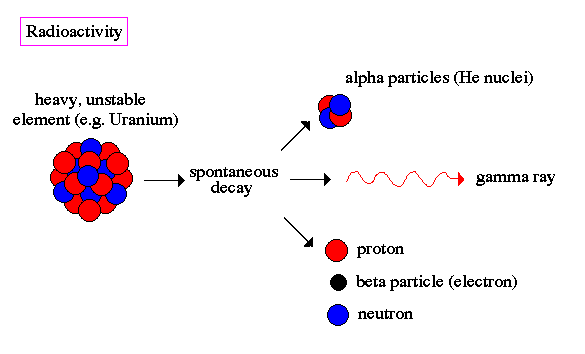
The alpha particle was heavy and positively charged, we now know that it is the helium nuclei (2 protons and 2 neutrons). The beta particle was light and negatively charged, the electron. Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. His experiment looked like the following:
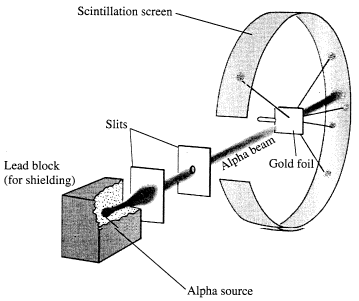
The Rutherford beamed alpha particles through gold foil and detected them as flashes of light or scintillations on a screen. The gold foil was only 0.00004 centimeter thick, meaning on a few hundreds of atoms thick.
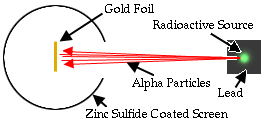
The expectation is that they will strike the fluorescent screen directly behind the foil.
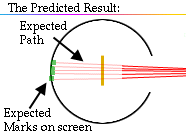
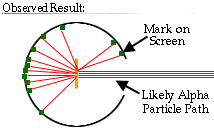
These results can best explained by a model for the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun.
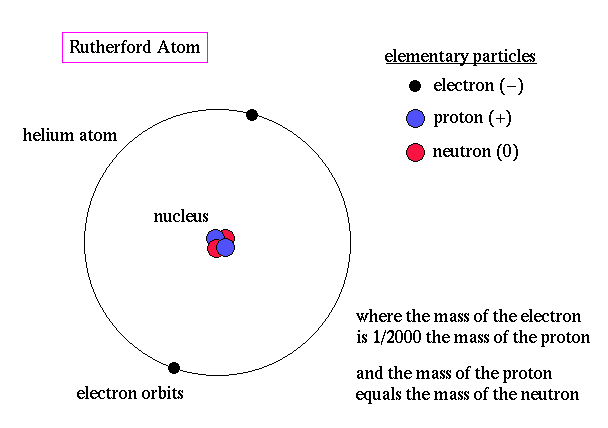
The Rutherford atomic model has been alternatively called the nuclear atom, or the planetary model of the atom.
Elementary Particles :
One of the primary goals in modern physics is to answer the question "What is the Universe made of?" Often that question reduces to "What is matter and what holds it together?" This continues the line of investigation started by Democritus, Dalton and Rutherford.
Modern physics speaks of fundamental building blocks of Nature, where fundamental takes on a reductionist meaning of simple and structureless. Many of the particles we have discussed so far appear simple in their properties. All electrons have the exact same characteristics (mass, charge, etc.), so we call an electron fundamental because they are all non-unique.
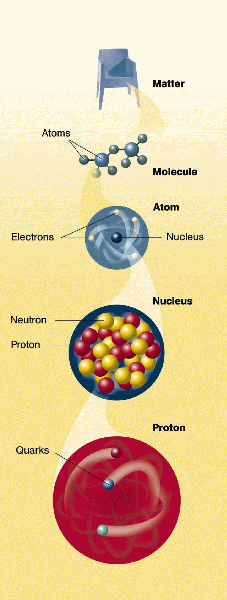
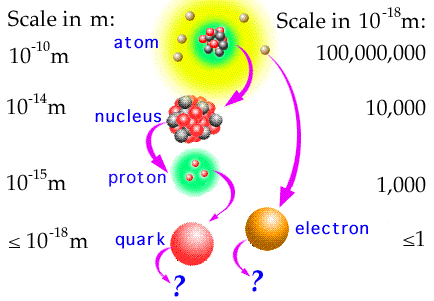
Quarks and Leptons:
The two most fundamental types of particles are quarks and leptons. The quarks and leptons are divided into 6 flavors corresponding to three generations of matter. Quarks (and antiquarks) have electric charges in units of 1/3 or 2/3's. Leptons have charges in units of 1 or 0.
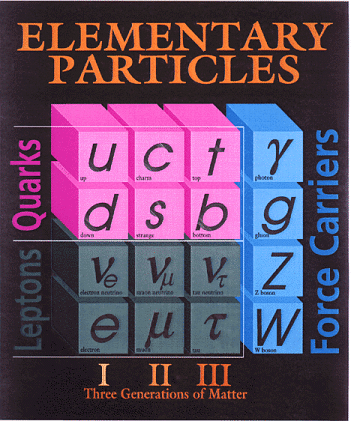
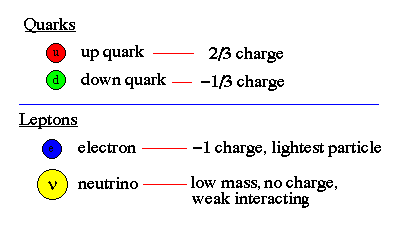
Baryons and Mesons:
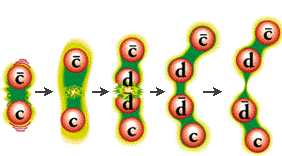
Quarks combine to form the basic building blocks of matter, baryons and mesons. Baryons are made of three quarks to form the protons and neutrons of atomic nuclei (and also anti-protons and anti-neutrons). Mesons, made of quark pairs, are usually found in cosmic rays. Notice that the quarks all combine to make charges of -1, 0, or +1.
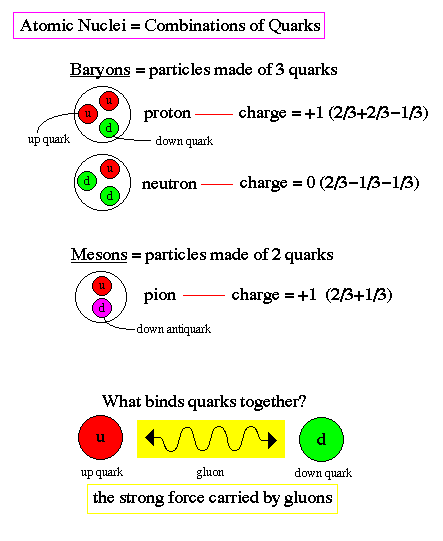
Quark Confinement:
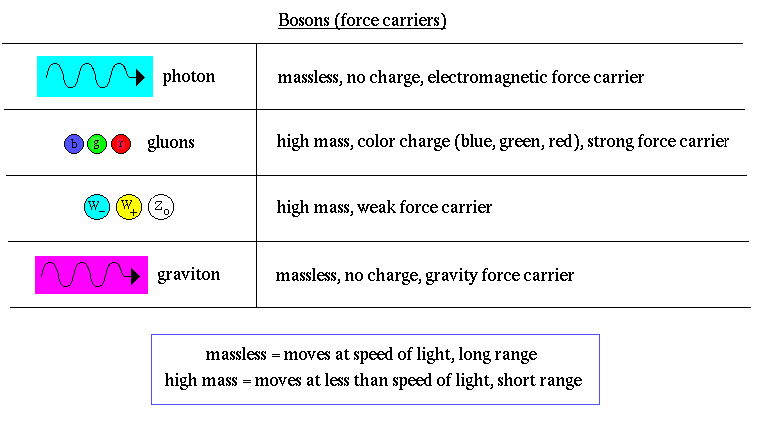
There can exist no free quarks, i.e. quarks by themselves. All quarks must be bound to another quark or antiquark by the exchange of gluons. This is called quark confinement. The exchange of gluons produces a color force field, referring to the assignment of color charge to quarks, similar to electric charge.
The color force field is unusual in that separating the quarks makes the force field stronger (unlike electromagnetic or gravity forces which weaken with distance). Energy is needed to overcome the color force field. That energy increases until a new quark or antiquark is formed (energy equals mass, E=mc2).
Theory of Everything:
Is that it? Are quarks and leptons the fundamental building blocks? Answer = maybe. We are still looking to fill some holes in what is know as the Standard Model.
The Standard Model is a way of making sense of the multiplicity of elementary particles and forces within a single scheme. The Standard Model is the combination of two schemes; the electroweak force (unification of electromagnetism and weak force) plus quantum chromodynamics. Although the Standard Model has brought a considerable amount of order to elementary particles and has led to important predictions, the model is not without some serious difficulties.
For example, the Standard Model contains a large number of arbitrary constants. Good choice of the constants leads to exact matches with experimental results. However, a good fundamental theory should be one where the constants are self-evident.
The Standard Model does not include the unification of all forces and, therefore, is incomplete. There is a strong expectation that there exists a Grand Unified Field Theory (GUTS) that will provide a deeper meaning to the Standard Model and explain the missing elements.
Supergravity:
Even a GUTS is incomplete because it would not include spacetime and therefore gravity. It is hypothesized that a ``Theory of Everything'' (TOE) will bring together all the fundamental forces, matter and curved spacetime under one unifying picture. For cosmology, this will be the single force that controlled the Universe at the time of formation. The current approach to the search for a TOE is to attempt to uncover some fundamental symmetry, perhaps a symmetry of symmetries. There should be predictions from a TOE, such as the existence of the Higgs particle, the origin of mass in the Universe.
One example of a attempt to formula a TOE is supergravity, a quantum theory that unities particle types through the use of ten dimensional spacetime (see diagram below). Spacetime (4D construct) was successful at explaining gravity. What if the subatomic world is also a geometric phenomenon.
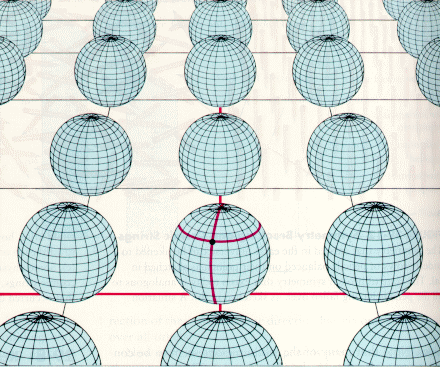
Many more dimensions of time and space could lie buried at the quantum level, outside our normal experience, only having an impact on the microscopic world of elementary particles.
It is entirely possible that beneath the quantum domain is a world of pure chaos, without any fixed laws or symmetries. One thing is obvious, that the more our efforts reach into the realm of fundamental laws, the more removed from experience are the results.
String Theory:
Another recent attempt to form a TOE is through M (for membrane) or string theory. String theory is actually a high order theory where other models, such as supergravity and quantum gravity, appear as approximations. The basic premise to string theory is that subatomic entities, such as quarks and forces, are actually tiny loops, strings and membranes that behave as particles at high energies.
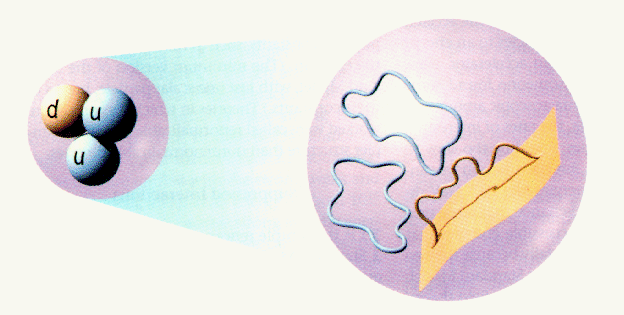
One of the problems in particle physics is the bewildering number of elementary particles (muons and pions and mesons etc). String theory answers this problem by proposing that small loops, about 100 billion billion times smaller than the proton, are vibrating below the subatomic level and each mode of vibration represents a distinct resonance which corresponds to a particular particle. Thus, if we could magnify a quantum particle we would see a tiny vibrating string or loop.
The fantastic aspect to string theory, that makes it such an attractive candidate for a TOE, is that it not only explains the nature of quantum particles but it also explains spacetime as well. Strings can break into smaller strings or combine to form larger strings. This complicated set of motions must obey self-consistent rules and the the constraint caused by these rules results in the same relations described by relativity theory.
Another aspect of string theory that differs from other TOE candidates is its high aesthetic beauty. For string theory is a geometric theory, one that, like general relativity, describes objects and interactions through the use of geometry and does not suffer from infinities or what is called normalization problems such as quantum mechanics. It may be impossible to test the predictions of string theory since it would require temperature and energies similar to those at the beginning of the Universe. Thus, we resort to judging the merit of this theory on its elegance and internal consistence rather than experiment data.