Interactive
Lecture Demonstrations
Prediction
Sheet—Heat & Phase Changes
Directions: Click here to download the prediction sheet on
which you will record your predictions. Write your name at the top to record
your presence and participation in these demonstrations. For each demonstration, record your
prediction(s) on this sheet before making any observations. You may be asked to send this sheet to your
instructor.
|
Demonstration
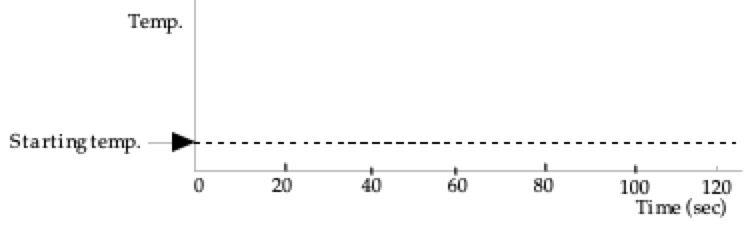
1: Water initially at room temperature is in a
perfectly insulated cup (no heat can leak in or out). During the first 20 seconds no heat is
transferred to the water, and then during the next 60 seconds, heat is
transferred at a steady rate. Then no
more heat is transferred. Sketch below
your prediction for the graph of the temperature of the water as a function
of time. Only after you have made your
prediction, click here to download and view
the video of the experiment. Compare the result with your prediction. (Note
that each Explain the shape of the three sections
of the graph: (1) no heat transferred, (2) heat transferred at a steady rate
and (3) no heat transferred. Why does the temperature rise at a
steady rate as the heat is transferred to the water at a steady rate? |
|
|
Demonstration
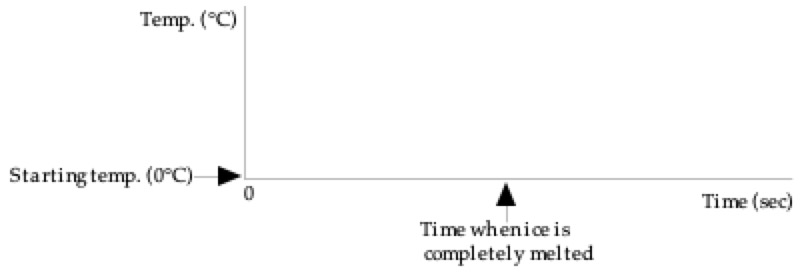
2: Heat is transferred at a steady rate to a
mixture of water and ice at 0°C in a perfectly insulated cup (no heat can
leak in or out). After the ice has
completely melted, heat is still transferred for awhile. Sketch below your prediction for the graph
of the temperature as a function of time. Only after you have made your
prediction, click here to download and view
the video of the experiment. Compare the result with your prediction. (Note
that each Is there any difference between the
shapes of the two sections of the graph: (1) before all the ice has melted,
and (2) after all the ice has melted. If they are
different in shape, describe any differences and explain why. Explain
what is happening to the heat transferred before the ice melts? |
|
|
Demonstration
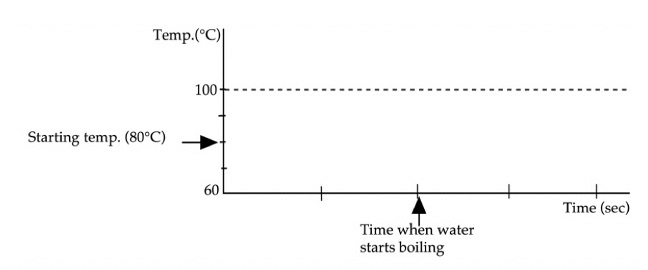
3: Heat is transferred at a steady rate to
water initially at 80°C in a perfectly insulated cup (no heat can leak in or
out). After the water starts boiling,
heat is still transferred for awhile. Sketch below
your prediction for the graph of the temperature as a function of time. Only after you have made your
prediction, click here to download and view
the video of the experiment. Compare the result with your prediction. (Note
that each Is there any difference between the
shapes of the two sections of the graph: (1) before the water begins boiling,
and (2) after the water is boiling. If they are
different in shape, describe any differences and explain why. Explain what is happening to the heat
transferred after the water is boiling? |
|