



Russell J. Donnelly
541-346-4226 (Tel)
541-346-5861 (Fax)
![]()
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |
![]()
![]() The
Observed Properties of Liquid Helium
The
Observed Properties of Liquid Helium
![]() at the Saturated Vapor
Pressure
at the Saturated Vapor
Pressure
Chapter 17. Saturated Vapor Pressure
Between
0.65 K and 5.0 K, T90 is defined in terms of the vapor pressure relations
of 3He and 4He. In this range, the interpolating
equation is of the form:
![]()
![]()
where P is the vapor pressure in Pa. For 4He the values of the coefficients
Ai and the constants B an C are given in Table 17.1. Although ITS-90 goes
onloy down to 0.65 K, this equation is,k in fact, valid to 0.5 K.
Table 17.1. Values of the coefficients in the equation for the saturated
vapor pressure for liquid 4He on the T90 scale.
4He 1.25 K to 2.1768 K |
4He 2.1768 K to 5.0 K |
|
| A0 | 1.392408 | 3.146631 |
| A1 | 0.527153 | 1.357655 |
| A2 | 0.166756 | 0.413923 |
| A3 | 0.050988 | 0.091159 |
| A4 | 0.026514 | 0.016349 |
| A5 | 0.001975 | 0.001826 |
| A6 | -0.017976 | -0.004325 |
| A7 | 0.005409 | -0.004973 |
| A8 | 0.013259 | 0.0 |
| A9 | 0.0 | 0.0 |
| B | 5.6 | 10.3 |
| C | 2.9 | 1.9 |
Below 1.25 K, the simple equation
![]()
![]()
suffices
to give P in terms of T or vice versa, where L0 = 59.83 J/mol is the latent
heat of evaporation at absolute zero,![]() , and R = 8.314510 J/mol•K.
, and R = 8.314510 J/mol•K.
Since the interpolating equation gives temperature as a function of pressure,
we offer a spline which returns the pressure as a fuction of temperature.
Table 17.2 gives the knots and coefficients.
Table 17.2. Knots and coefficients for the saturated vapor pressure of
liquid 4He on T90.
Knots |
Coefficients |
Knots |
Coefficients |
| K(1)=0.5 | C(1)=0.00183797 | K(18)=1.5 | C(18)=1886.00 |
| K(2)=0.5 | C(2)=0.00250000 | K(19)=1.7 | C(19)=3194.00 |
| K(3)=0.5 | C(3)=0.00376000 | K(20)=1.85 | C(20)=5569.50 |
| K(4)=0.5 | C(4)=0.00624000 | K(21)=2 | C(21)=9279.10 |
| K(5)=0.52 | C(5)=0.0123600 | K(22)=2.2 | C(22)=15370.0 |
| K(6)=0.53 | C(6)=0.0330600 | K(23)=2.5 | C(23)=23480.0 |
| K(7)=0.56 | C(7)=0.0933900 | K(24)=2.7 | C(24)=37355.0 |
| K(8)=0.6 | C(8)=0.258560 | K(25)=3 | C(25)=57050.0 |
| K(9)=0.65 | C(9)=0.626900 | K(26)=3.3 | C(26)=87170.0 |
| K(10)=0.7 | C(10)=1.37025 | K(27)=3.7 | C(27)=132825 |
| K(11)=0.75 | C(11)=2.93305 | K(28)=4.05 | C(28)=179650 |
| K(12)=0.8 | C(12)=6.37318 | K(29)=4.5 | C(29)=211567 |
| K(13)=0.85 | C(13)=15.0042 | K(30)=5.1 | |
| K(14)=0.92 | C(14)=38.0570 | K(31)=5.1 | |
| K(15)=1 | C(15)=116.050 | K(32)=5.1 | |
| K(16)=1.1 | C(16)=362.700 | K(33)=5.1 | |
| K(17)=1.25 | C(17)=990.900 |
![]()
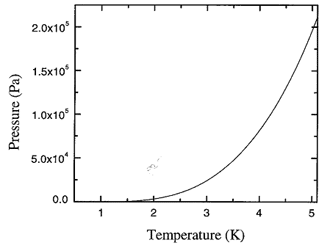
Figure 17.1. The recommended values for the saturated vapor pressure (in Pa) of liquid 4He calculated from the spline
![]()
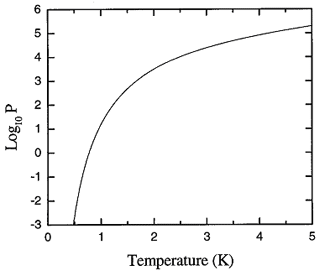
Figure 17.2. Log10 of the saturated vapor pressure curve (in Pa) of liquid 4He.
Table 17.3. Recommended values of the saturated vapor pressure of liquid 4He
T90 (K) |
P (Pa) |
T90 (K) |
P (Pa) |
| 0.650 | 1.101E-01 | 2.900 | 2.063E+04 |
| 0.700 | 2.923E-01 | 2.950 | 2.229E+04 |
| 0.750 | 6.893E-01 | 3.000 | 2.405E+04 |
| 0.800 | 1.475E+00 | 3.050 | 2.589E+04 |
| 0.850 | 2.914E+00 | 3.100 | 2.783E+04 |
| 0.900 | 5.380E+00 | 3.150 | 2.987E+04 |
| 0.950 | 9.381E+00 | 3.200 | 3.201E+04 |
| 1.000 | 1.558E+01 | 3.250 | 3.425E+04 |
| 1.050 | 2.479E+01 | 3.300 | 3.659E+04 |
| 1.100 | 3.802E+01 | 3.350 | 3.904E+04 |
| 1.150 | 5.647E+01 | 3.400 | 4.160E+04 |
| 1.200 | 8.152E+01 | 3.450 | 4.426E+04 |
| 1.250 | 1.147E+02 | 3.500 | 4.705E+04 |
| 1.300 | 1.579E+02 | 3.550 | 4.994E+04 |
| 1.350 | 2.129E+02 | 3.600 | 5.296E+04 |
| 1.400 | 2.819E+02 | 3.650 | 5.609E+04 |
| 1.450 | 3.673E+02 | 3.700 | 5.935E+04 |
| 1.500 | 4.715E+02 | 3.750 | 6.273E+04 |
| 1.550 | 5.971E+02 | 3.800 | 6.625E+04 |
| 1.600 | 7.465E+02 | 3.850 | 6.989E+04 |
| 1.650 | 9.226E+02 | 3.900 | 7.366E+04 |
| 1.700 | 1.128E+03 | 3.950 | 7.757E+04 |
| 1.750 | 1.366E+03 | 4.000 | 8.162E+04 |
| 1.800 | 1.638E+03 | 4.050 | 8.580E+04 |
| 1.850 | 1.949E+03 | 4.100 | 9.013E+04 |
| 1.900 | 2.299E+03 | 4.150 | 9.461E+04 |
| 1.950 | 2.692E+03 | 4.200 | 9.923E+04 |
| 2.000 | 3.130E+03 | 4.250 | 1.040E+05 |
| 2.050 | 3.613E+03 | 4.300 | 1.089E+05 |
| 2.100 | 4.141E+03 | 4.350 | 1.140E+05 |
| 2.150 | 4.716E+03 | 4.400 | 1.193E+05 |
| 2.200 | 5.335E+03 | 4.450 | 1.247E+05 |
| 2.250 | 6.005E+03 | 4.500 | 1.303E+05 |
| 2.300 | 6.730E+03 | 4.550 | 1.360E+05 |
| 2.350 | 7.512E+03 | 4.600 | 1.419E+05 |
| 2.400 | 8.354E+03 | 4.650 | 1.480E+05 |
| 2.450 | 9.258E+03 | 4.700 | 1.543E+05 |
| 2.500 | 1.023E+04 | 4.750 | 1.608E+05 |
| 2.550 | 1.127E+04 | 4.800 | 1.674E+05 |
| 2.600 | 1.237E+04 | 4.850 | 1.743E+05 |
| 2.650 | 1.355E+04 | 4.900 | 1.813E+05 |
| 2.700 | 1.481E+04 | 4.950 | 1.886E+05 |
| 2.750 | 1.614E+04 | 5.000 | 1.960E+05 |
| 2.800 | 1.755E+04 | 5.050 | 2.037E+05 |
| 2.850 | 1.905E+04 | 5.100 | 2.116E+05 |
![]()
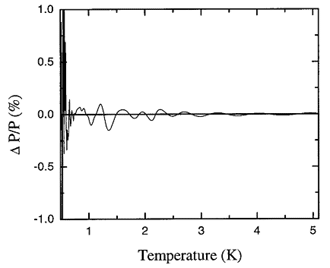
Figure 17.3. The fractional deviation of values of the saturated vapor pressure of 4He calculated with the spline from those calculated with the ITS-90 equations expressed as percent.
It
is possible that one might want to know the vapor pressure on the T58
scale since the bulk of available data has been taken on T58. Table 17.4
gives the knots and coefficients of a spline which returns the log10 of
the saturated vapor pressure in ![]() Hg
(the usual unit for T58).
Hg
(the usual unit for T58). ![]() Hg
= 0.1332 Pa.
Hg
= 0.1332 Pa.
Table
17.4. Knots and coefficients of a spline which returns the log10 of the
saturated vapor pressure.
Knots |
Coefficients |
| K(1)=1.000000 | C(1)=2.079205 |
| K(2)=1.000000 | C(2)=2.290418 |
| K(3)=1.000000 | C(3)=2.641180 |
| K(4)=1.000000 | C(4)=3.067643 |
| K(5)=1.151055 | C(5)=3.434872 |
| K(6)=1.287657 | C(6)=3.744602 |
| K(7)=1.432021 | C(7)=4.016736 |
| K(8)=1.598327 | C(8)=4.269063 |
| K(9)=1.740550 | C(9)=4.453011 |
| K(10)=1.899594 | C(10)=4.558079 |
| K(11)=2.101790 | C(11)=4.624146 |
| K(12)=2.176014 | C(12)=4.760414 |
| K(13)=2.183220 | C(13)=4.923719 |
| K(14)=2.288467 | C(14)=5.078931 |
| K(15)=2.579378 | C(15)=5.188290 |
| K(16)=2.740483 | C(16)=5.287068 |
| K(17)=2.889599 | C(17)=5.379486 |
| K(18)=3.040574 | C(18)=5.463746 |
| K(19)=3.189656 | C(19)=5.543383 |
| K(20)=3.340566 | C(20)=5.618267 |
| K(21)=3.479662 | C(21)=5.689276 |
| K(22)=3.630567 | C(22)=5.756536 |
| K(23)=3.779660 | C(23)=5.820032 |
| K(24)=3.920276 | C(24)=5.861069 |
| K(25)=4.070000 | C(25)=5.880814 |
| K(26)=4.215000 | |
| K(27)=4.215000 | |
| K(28)=4.215000 | |
| K(29)=4.215000 |
![]()
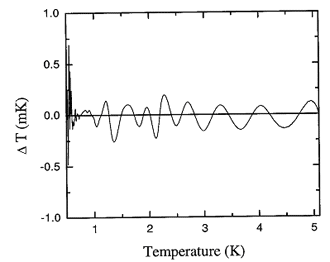
Figure 17.4. Deviations in temperature ?T corresponding to the deviations in pressure shown in Figure 17.3.
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |

