![]()
The root source of energy used for heat, transportation, and industry in most of the world is chemical energy. This type of energy constitutes the energy that can be gained by breaking chemical bonds and rearranging the molecules of wood, coal, natural gas and petroleum-- to name the most popular fuels-- into other molecules by a process called oxidation (or "burning").
Typical atoms measure 0.1 nanometer (.0000000001 meters) in size. Size, remember, is defined by the "orbits" of electrons relative to a nucleus comprising protons and neutrons:
- Figure 1: The Bohr hydrogen atom
The attractive force between the positively-charged protons in the nucleus and the negatively-charged electrons hold electrons in their "orbits." Also, like-charged electrons repel each other via the electric force. Remember that the proton and the electron have the same charge, just with opposite signs. The electron mass, however, is 1/1830 that of the proton. This means that the electron moves much more readily than the proton, even though the magnitude of the electric force on each is the same.
In the classical view, we can imagine atoms as tiny negative-charged electrons circling around a massive nucleus comprising positive-charged protons and neutrons. If an atom is not ionized, it has no net charge- hence the total charge of the electrons just balances that of all the protons in the nucleus.
Atoms emit or absorb EM radiation (light, IR, UV radiation, etc.) when electrons change orbits about an atom.

Let's take a look at a video segment that explains this.
If we excite neon atoms by colliding electrons off of them, we have a neon light. Electrons changing orbits about the neon atoms will cause EM radiation to be emitted, some as visible light. What colors of light do we expect to see? Why?
In real atoms the electrons can not all get close to the oppositely-charged nucleus. The experimentally-verified Pauli exclusion principle states that no two electrons may occupy the same state (defined by their orbit, energies, etc.).
- Figure 2: Electron states
A consequence of the Pauli exclusion principle and a branch of physics called quantum mechanics is that electrons take certain average orbits around atomic nuclei. The first electrons occupy the lowest state (closest average position to the nucleus), others fill shells (defined by their average radius from the nucleus and the orbital angular momentum associated with them), progressing from closest to further away from the nucleus.
Electrons, you say! Well, one other property of electrons, their spin, distinguishes one from another. Electrons with spin up can occupy the same shell as spin-down electrons. Thus each shell as defined by its energy level, angular momentum, mean distance from the nucleus, etc., can contain two electrons.
- Figure 3: Electron spin
Each of these shells has a certain energy associated with it. Electrons in the innermost shells have given up the most energy to get there. They are said to be in a stable energy level. Figure 4 shows the average radii for the first three orbits of a simple hydrogen atom. As one progresses outward from the nucleus, it is easier and easier to dislodge an electron from its orbit.
For example for the Bohr model of hydrogen, one need only input 1.5 eV (electron Volt, another unit of energy) to dislodge an electron in the n=3 orbit, while it takes 13.6 eV to strip an electron in the innermost orbit.
Figure 4: The Bohr model of the hydrogen atom
(Source: Energy by G. Aubrecht)
How do we know that electrons can only change between certain "states?" For our ILD we observed only certain colors of light, representing the energy intervals (and, hence colors) between the allowed states for neon atom electrons.
Electrons moving in circular orbits at constant speed around a nucleus (which is actually an approximation of reality) are said to exhibit periodic motion. This is the same motion exhibited by a mass attached to a spring, a pendulum, or the motion of small segments of a violin string when it is bowed. To see this, let's look at Figure 5, below, which compares motion about a circle to a standing wave on a string.
Figure 5: a) body moving a constant speed (NOT velocity) around a circle (web browser-willing...); b) motion viewed edge-on; c) motion of a segment of a standing wave on a rope (lower right).
Let's compare the motion of the body moving around the circle to that of a segment of a stretched rope undergoing oscillation. One can see (Figure 5) that the motion of the body, when viewed edge on, is identical to that of a segment of the oscillating rope.
Note, also, that the oscillating rope appears to prefer certain types of motion at the actively moving end. More specifically, if I wiggle the end of the rope up and down just right, waves appear to stand still on the rope. If I wiggle up and down more vigorously but at just the right frequency (number of times wiggled upwards per second), one can see another, shorter standing wave on the rope. Note that the ribbon tied to the rope moves exactly as did the body around the circle, when it was viewed on edge.
It turns out the electrons in their orbits around atomic nuclei exhibit the same behavior. That is, only certain frequencies of oscillation are allowed. There is an energy level associated with each frequency. The higher the frequency, the higher the energy. Frequency (color) is proportional to energy:
It may not be obvious to you, but when I wiggle this rope trying to excite a higher frequency standing wave, I am certainly converting more energy in the process. All other things being equal, blue light has more (electromagnetic) energy than does red. Infrared light, which the "heat light" in your bathroom emits, has less energy than do x-rays of equal amplitude.
Atoms generally absorb and emit energy as quanta, discrete amounts of energy. How does this work? In words, atoms emit or absorb energy when one of their electrons changes state-- which means it changes to a different orbit, or otherwise changes angular momentum. For example atoms emit energy, say as light, when one of their electrons lowers its orbit. An incoming photon, or bit of light, can cause an orbiting electron to increase its orbit or its angular momentum.

Let's finish our video segment that explains this.
![]()
Three important things to remember:
How is this important for the environment? The sun emits light at many wavelengths, including infrared, visible and ultraviolet. 34% of that light which reaches the Earth is reflected off the atmosphere, clouds, dust, the Earth's surface, and greenhouse gases such as CO2. Approximately 64% of the remaining light is absorbed by the Earth and its oceans, heating them up. They, in turn, emit low-energy infrared light, some of which is prevented from escaping the atmosphere by greenhouse gases. All of these processes, reflection, absorption and reflection of infrared from the Earth involve the atomic changes that have been described above.

Remember our graph showing the spectrum of sunlight passing through the Earth's atomsphere?
Figure 6: Absorption spectrum of sunlight by Earth's atmosphere
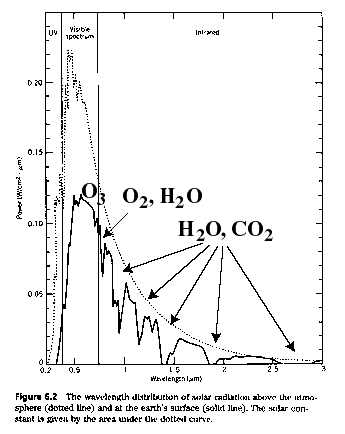
(source: Energy & Problems of a Tech. Society- Kraushaar & Ristenen, absorption lines adopted from Piexoto & Oort, Physics of Climate)
Well, those dips in the spectrum are where certain molecules (e.g., CO2) are aborbing light. Certain gases (again, CO2) absorb infrared (IR) light better than other "colors." Thus, they absorb IR "light" emitted by the Earth as it is heated by the sun. This effectively keeps that thermal energy within our atmosphere and prevents it from escaping to space. Voila the greenhouse effect!
![]()
Different atoms have different numbers of electrons, protons and neutrons. The number of protons in an atom gives its atomic number. For example helium has two protons (and two electrons), so its atomic number is 2.
Because electrons fill shells, and each shell can hold a certain number of electrons, different atoms (hydrogen, helium, carbon, etc.) have their outer shells partially filled with electrons.
Atoms of some elements, such as helium or argon, have completely filled outer shells. They are sometimes called inert because it is very difficult to get them to react (share electrons with) other elements.
The periodic table of the elements shows atomic numbers and how shells are filled with electrons for all the elements. For example, elements in column 1A (e.g., Na=sodium) all have a filled shell plus one, extra electron. Consequently, it is easy for those atoms to give up that one electron. Elements in column VIIA (e.g., Cl=chlorine) are missing one electron in their outermost shell. Thus they bond well with elements from column 1A. For example, NaCl, the molecule with a sodium and a chlorine atom, is otherwise known as table salt.
When Na is combined with Cl, energy is given off. This type of reaction is called exothermic. Other reactions use up more energy in making molecular bonds than they give off. They are called endothermic reactions. In most cases some energy is needed to start a reaction, even if that reaction is ultimately exothermic. This "starter energy" is called the activation energy. For example, the energy liberated in lighting a match to start a fire is activation energy.
Once an exothermic reaction is started, some of the liberated, thermal energy can go towards activating the reaction elsewhere. Ultimately all the original molecules will be reorganized into byproduct molecules and the reaction must stop.
When certain chemicals-- those containing carbon in some form (typically attached to hydrogen atoms)-- undergo chemical reactions with oxygen, they are said to be burned. A typical byproduct of such a reaction is CO2. The (thermal) energy liberated by such a chemical process is quantified by the fuel's heat of combustion-- the amount of energy given off per kilogram of fuel. Remember how we compared the heat of combustion for various fossil fuels, butter, wood, etc. during the last lecture.
Certain molecules, when added to a chemical reaction, can increase the rate at which that reaction takes place. They do this by "holding" the constituents in such a way that each atoms unfilled electron shells are in close proximity to one another. These catalyst molecules are not used up during the chemical reaction.
Certain kinds of catalysts found in living things are referred to as enzymes-- proteins that act to speed up chemical reactions that serve to fuel living things. Photosynthesis-- the process whereby plant cells absorb energy from sunlight to allow an endothermic reaction of water and carbon dioxide to take place-- proceeds by first using enzymes to make oxygen out of carbon dioxide. Ultimately sunlight allows hydrogen atoms to react with carbon dioxide to form sugars. The "heat of combustion" for sugars typically fixed by photosynthesis is about 6.2 kJ/kg.