The ancient philosopher, Heraclitus, maintained that everything is in a state of flux. Nothing escapes change of some sort (it is impossible to step into the same river). On the other hand, Parmenides argued that everything is what it is, so that it cannot become what is not (change is impossible because a substance would have to transition through nothing to become something else, which is a logical contradiction). Thus, change is incompatible with being so that only the permanent aspects of the Universe could be considered real.
An ingenious escape was proposed in the fifth century B.C. by Democritus. He hypothesized that all matter is composed of tiny indestructible units, called atoms. The atoms themselves remain unchanged, but move about in space to combine in various ways to form all macroscopic objects. Early atomic theory stated that the characteristics of an object are determined by the shape of its atoms. So, for example, sweet things are made of smooth atoms, bitter things are made of sharp atoms.
In this manner permanence and flux are reconciled and the field of atomic physics was born. Although Democritus' ideas were to solve a philosophical dilemma, the fact that there is some underlying, elemental substance to the Universe is a primary driver in modern physics, the search for the ultimate subatomic particle.

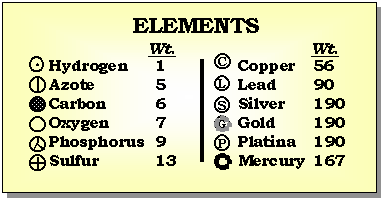
It was John Dalton, in the early 1800's, who determined that each chemical element is composed of a unique type of atom, and that the atoms differed by their masses. He devised a system of chemical symbols and, having ascertained the relative weights of atoms, arranged them into a table. In addition, he formulated the theory that a chemical combination of different elements occurs in simple numerical ratios by weight, which led to the development of the laws of definite and multiple proportions.
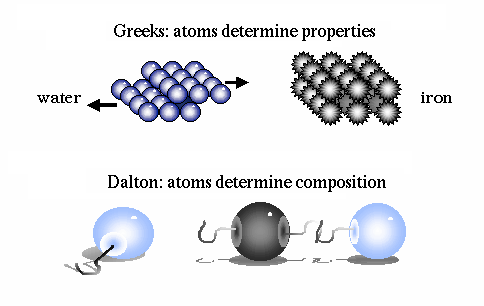
He then determined that compounds are made of molecules, and that molecules are composed of atoms in definite proportions. Thus, atoms determine the composition of matter, and compounds can be broken down into their individual elements.

The first estimates for the sizes of atoms and the number of atoms per unit volume where made by Joesph Loschmidt in 1865. Using the ideas of kinetic theory, the idea that the properties of a gas are due to the motion of the atoms that compose it, Loschmidt calculated the mean free path of an atom based on diffusion rates. His result was that there are 6.022x1023 atoms per 12 grams of carbon. And that the typical diameters of an atom is 10-8 centimeters.
Matter:
Matter exists in four states: solid, liquid, gas and plasma. Plasmas are only found in the coronae and cores of stars. The state of matter is determined by the strength of the bonds between the atoms that makes up matter. Thus, is proportional to the temperature or the amount of energy contained by the matter.
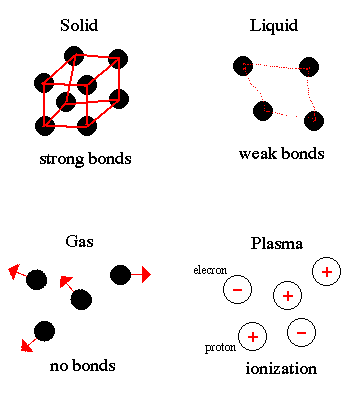
The change from one state of matter to another is called a phase transition. For example, ice (solid water) converts (melts) into liquid water as energy is added. Continue adding energy and the water boils to steam (gaseous water) then, at several million degrees, breaks down into its component atoms.
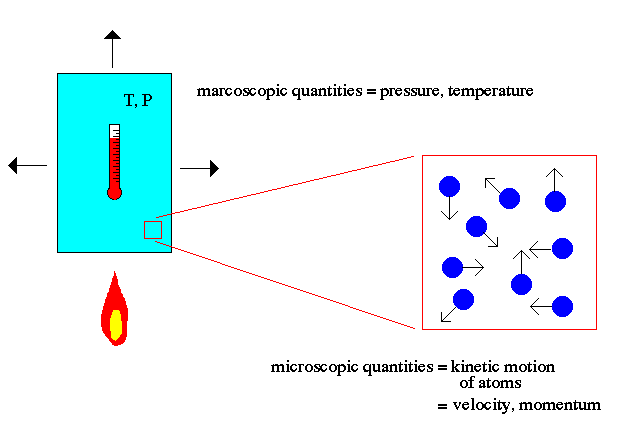
Atomic theory is the field of physics that describes the characteristics and properties of atoms that make up matter. The key point to note about atomic theory is the relationship between the macroscopic world (us) and the microscopic world of atoms. For example, the macroscopic world deals with concepts such as temperature and pressure to describe matter. The microscopic world of atomic theory deals with the kinetic motion of atoms to explain macroscopic quantities.
Temperature is explained in atomic theory as the motion of the atoms (faster = hotter). Pressure is explained as the momentum transfer of those moving atoms on the walls of the container (faster atoms = higher temperature = more momentum/hits = higher pressure).
Thomson Atom:
The next great step forward in the understanding of atoms was accomplished by John Thomson. Using a cathode ray scope, Thomson determined that all matter, whatever its source, contains particles of the same kind that are much less massive than the atoms of which they form a part. They are now called electrons, although he originally called them corpuscles.
His discovery was the result of an attempt to solve a long-standing controversy regarding the nature of cathode rays, which occur when an electric current is driven through a vessel from which most of the air or other gas has been pumped out.
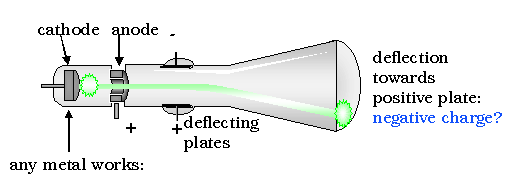
By applying an improved vacuum technique, Thomson was able to put forward a convincing argument that these rays were composed of particles. Furthermore, these rays seemed to be composed of the same particles regardless of what kind of gas carried the electric discharge or what kinds of metals were used as conductors.
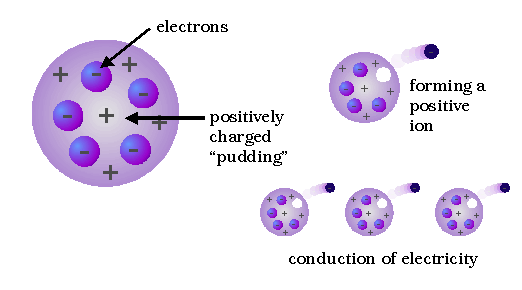
Thomson's conclusion that the electrons were present in all kinds of matter was strengthened during the next three years, when he found that electrons with the same properties could be produced in other ways; e.g., from hot metals. Thomson may be described as "the man who split the atom" for the first time, although "chipped" might be a better word, in view of the size and number of electrons.
Rutherford Atom:
Ernest Rutherford is considered the father of nuclear physics. Indeed, it could be said that Rutherford invented the very language to describe the theoretical concepts of the atom and the phenomenon of radioactivity. Particles named and characterized by him include the alpha particle, beta particle (which turned out to be Thomson's electron) and proton. Rutherford overturned Thomson's atom model in 1911 with his well-known gold foil experiment in which he demonstrated that the atom has a tiny, massive nucleus.
By the turn of the 20th century, physicists knew that certain elements emitted fast moving particles of two flavors, alpha particles and beta particles. These elements were typically very heavy (i.e. their atom nuclei were massive) such as uranium and radium. Today we know that heavy nuclei are unstable and `decay', meaning that they spontaneously split into smaller nuclei and emit stray particles. This is called radioactivity.
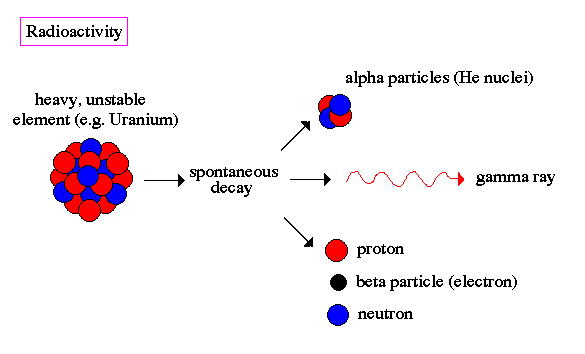
The alpha particle was heavy and positively charged, we now know that it is the helium nuclei (2 protons and 2 neutrons). The beta particle was light and negatively charged, the electron. Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. His experiment looked like the following:
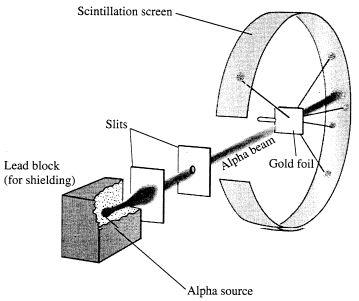
The Rutherford beamed alpha particles through gold foil and detected them as flashes of light or scintillations on a screen. The gold foil was only 0.00004 centimeter thick, meaning on a few hundreds of atoms thick.
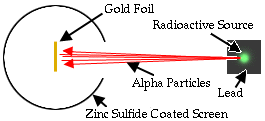
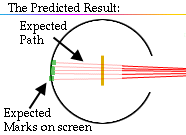
The expectation is that they will strike the fluorescent screen directly behind the foil.
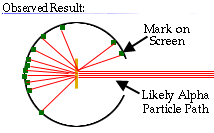
These results can best explained by a model for the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun.
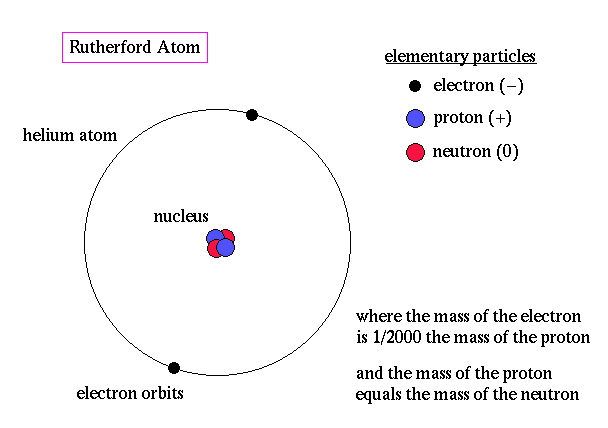
The Rutherford atomic model has been alternatively called the nuclear atom, or the planetary model of the atom.
Spectroscopy:
The fact that the temperature and energy generation of an object can be determined by its Planck curve made the study of spectrum the key component to stellar astronomy. The amount of energy emitted from stars is determined by measuring their brightness or the amount of light they emit. This is called photometry. However, two major developments expanded our understanding of the chemical make-up of stars. They were:
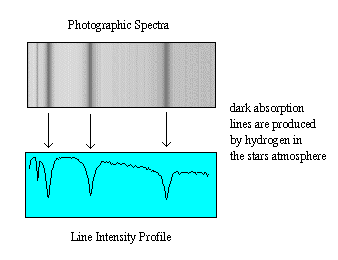
The discovery of spectra lines was made by Fraunhofer who, in the early 1800's, magnified the Sun's spectrum and discovered dark lines which could be identified with particular elements (based on spectra in the laboratories).

English astronomer Lockyer, in the late-1800's, discovered an unknown element in the Sun, i.e. a set of spectral lines which did not correspond to elements in the lab. He named this element helium (Latin for Sun element).

By the 20th century, spectral lines for all the elements in the periodic table have been classified and astronomers could examine the chemical composition of stars and planets. What was missing was an explanation for why these lines should exist. Why did particular atoms absorb photons at particular wavelengths?
Planck's constant:
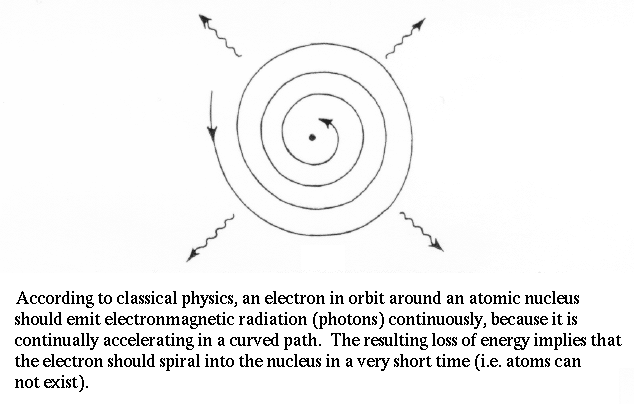
The dilemma of spectral lines presented serious problems for attempts to understand how light and matter interact. Planck also noticed another fatal flaw in our physics by demonstrating that the electron in orbit around the nucleus accelerates. Acceleration means a changing electric field (the electron has charge), when means photons should be emitted. But, then the electron would lose energy and fall into the nucleus. Therefore, atoms shouldn't exist!
To resolve this problem, Planck made a wild assumption that energy, at the sub-atomic level, can only be transferred in small units, called quanta. Due to his insight, we call this unit Planck's constant (h). The word quantum derives from quantity and refers to a small packet of action or process, the smallest unit of either that can be associated with a single event in the microscopic world.
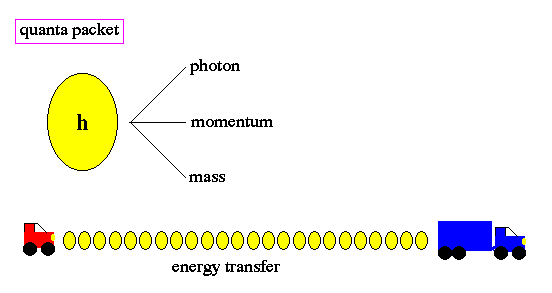
Changes of energy, such as the transition of an electron from one orbit to another around the nucleus of an atom, is done in discrete quanta. Quanta are not divisible. The term quantum leap refers to the abrupt movement from one discrete energy level to another, with no smooth transition. There is no ``inbetween''.
The quantization, or ``jumpiness'' of action as depicted in quantum physics differs sharply from classical physics which represented motion as smooth, continuous change.
Bohr Atom:
Perhaps the foremost scientists of the 20th century was Niels Bohr, the first to apply Planck's quantum idea to problems in atomic physics. In the early 1900's, Bohr proposed a quantum mechanical description of the atom to replace the early model of Rutherford.

The Bohr model basically assigned discrete orbits for the electron, multiples of Planck's constant, rather than allowing a continuum of energies as allowed by classical physics.
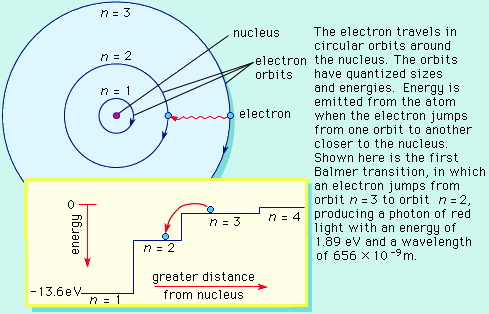
The power in the Bohr model was its ability to predict the spectra of light emitted by atoms. In particular, its ability to explain the spectral lines of atoms as the absorption and emission of photons by the electrons in quantized orbits.

In the above example, a beam of photons (light) contains many colors (wavelength = energy). Low energy photons are red, high energy are violet. As the photons pass through an atom, the orange photon with energy 2 matches the energy needed to kick an electron from the 0 state to the 2 state, and the photon is absorbed to do this. At the same time, the yellow photon of energy 3 stimulates the electron at state 5 to fall to state 2, emitting energy as an extra yellow photon of energy 3. The results to the beam of light is a missing photon 2 (absorbtion line) and an extra photon 3 (emission line).
Our current understanding of atomic structure was formalized by Heisenberg and Schroedinger in the mid-1920's where the discreteness of the allowed energy states emerges from more general aspects, rather than imposed as in Bohr's model. The Heisenberg/Schroedinger quantum mechanics have consistent fundamental principles, such as the wave character of matter and the incorporation of the uncertainty principle.
In principle, all of atomic and molecular physics, including the structure of atoms and their dynamics, the periodic table of elements and their chemical behavior, as well as the spectroscopic, electrical, and other physical properties of atoms and molecules, can be accounted for by quantum mechanics => fundamental science.

|
|

|