Roemer (1680's) was the first to measure the speed of light using Jupiter's moons -> c=299,790 km/sec or about 185,000 mi/sec
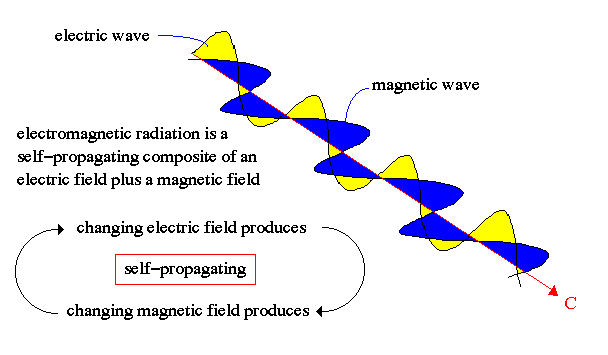
Maxwell (1850's) showed that light is energy carried in the form of opposite but supporting electric and magnetic fields in the shape of waves, i.e. self-propagating electromagnetic waves.

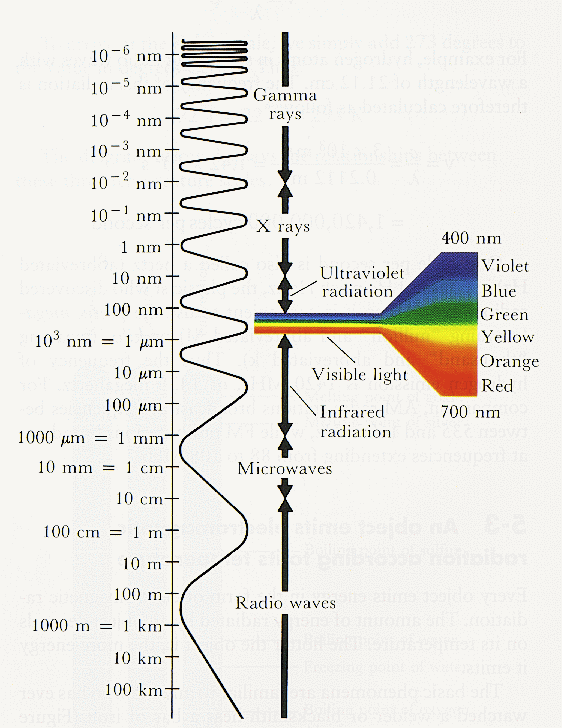
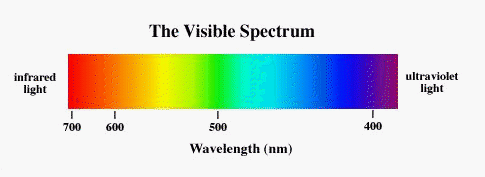
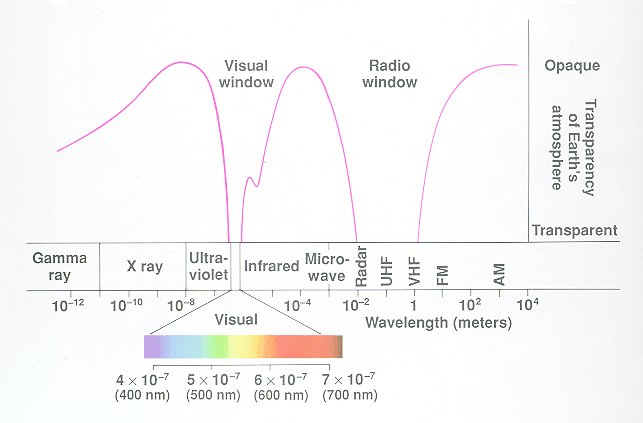
However, note that observing at different wavelengths requires vastly different technology and conditions. In particular, our atmosphere is opaque to certain wavelengths (good for us) meaning that they can only be observed from space (expensive for astronomers).
Wave Properties:
Due to its wave-like nature, light has three properties when encountering a medium:
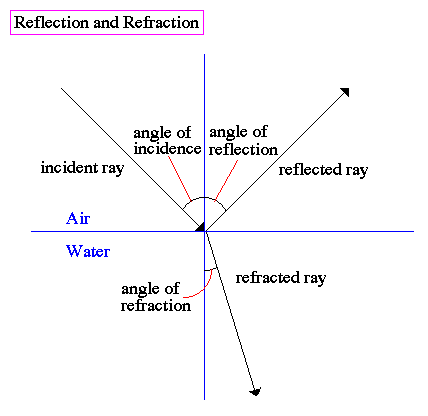
1) reflection
2) refraction
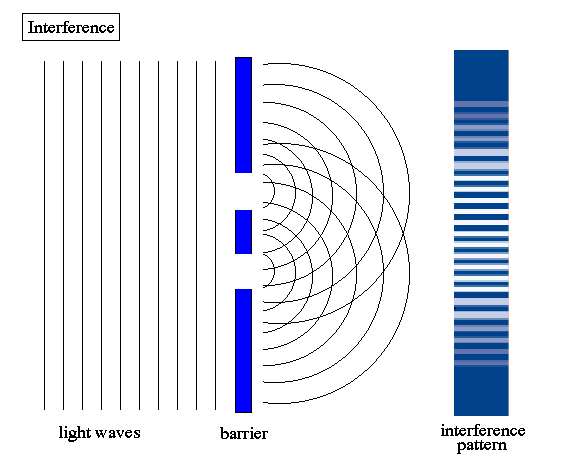
3) diffraction
When a light ray strikes a medium, such as oil or water, the ray is both refracted and reflected as shown below:
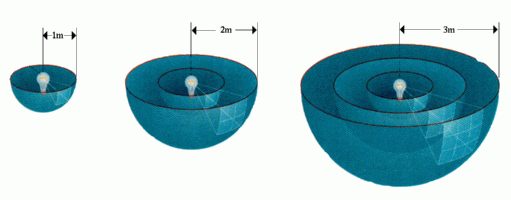
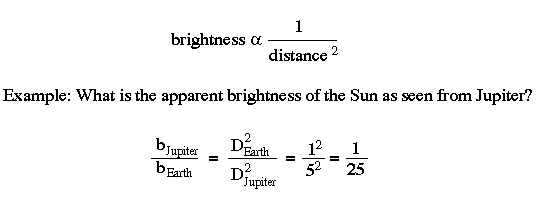
Inverse Square Law:
The brightness of an object varies inversely as the square of the distance. This is a geometric consequence of the fact that light moves outward in a spherical fashion.
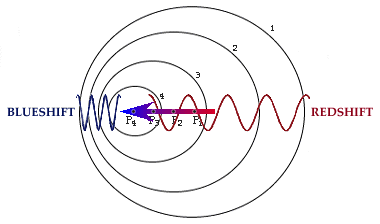
Doppler effect:
The Doppler effect occurs when on object that is emitting light is in motion with respect to the observer. The speed of light does not change, only the wavelength. If the object is moving towards the observer the light is ``compressed'' or blueshifted. If the object is moving away from the observer the light is ``expanded'' or redshifted.
Planck's curve:
One of the primary results from the field of spectroscopy was the discovery of how the energy outputed by an object (its spectrum) changes with temperature. In particular, was the formulation of the laws of radiation commonly expressed as two laws:
The energy outputted by an object (a star, a piece of metal, a human body) takes on a particular shape called Planck's curve, shown in the following plot of energy versus wavelength.
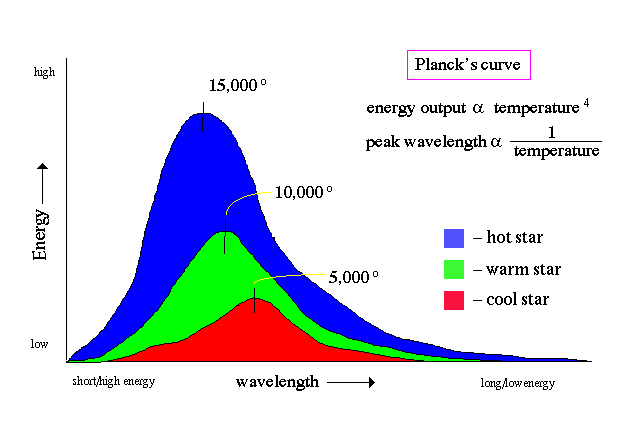
Notice that all objects emit all kinds of electromagnetic radiation. Except, cool objects (like humans) emit very little at short wavelengths (x-rays) and long wavelengths (radio). Most of our energy comes out in the infrared (our peak emission is at 10 microns).
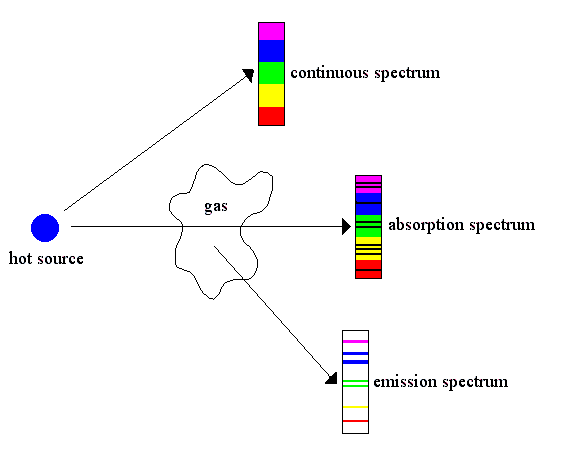
Kirchhoff's Laws:
There are three types of spectra emitted by objects:
1) Continuous spectrum - a solid or liquid body radiates an uninterrupted, smooth spectrum (Planck curve)
2) Emission spectrum - a radiating gas produces a spectrum of discrete spectral lines
3) Absorption spectrum - a continuous spectrum that passes through a cool gas has specific spectral lines removed (inverse of an emission spectrum)

|
|

|