Previous Projects
Myotonic Dystrophy

In a close collaboration with UO biochemist Andy Berglund and his lab, we developed therapeutics for Myotonic Dystrophy (DM). DM is the most common form of adult onset muscular dystrophy, affecting 1 in 8000 people. DM is caused by an expansion of three or four nucleotide repeats–CUG expansions in patients with myotonic dystrophy type 1 (DM1), and CCUG expansions in patients with myotonic dystrophy type 2 (DM2). Both DM1 and DM2 patients display many of the same symptoms (myotonia, progressive muscle weakness and wasting, cataracts, cardiac and developmental defects) strongly suggesting that both DM1 and DM2 progress through the same or very similar mechanisms.
In 2007 the Berglund lab discovered that pentamidine (1) will preferentially bind CUG repeats over other nucleotides and help to reverse some of the molecular defects of DM. We prepared a wide variety of pentamidine analogues (2-4) for determining structure-activity relationships to improve efficacy and decrease toxicity versus 1 and thus potentially generate a therapeutic for DM, in addition to providing additional tools to aid in the understanding of the roles of CUG repeats in DM progression.
Relevant Publications
(1) Khalifa, M. K.; Bodner, M. J.; Berglund, J. A.; Haley, M. M. Tetrahedron Lett. 2015, 56, 4109-4111.
(2) Siboni, R. B.; Bodner, M. J.; Khalifa, M. J.; Docter, A. G.; Choi, J. Y.; Nakamuri, M.; Haley, M. M.; Berglund, J. A. J. Med. Chem. 2015, 58, 5770-5780.
(3) Coonrod, L. A.; Nakamori, M.; Wang, W.; Carrell, S.; Hilton, C. L.; Bodner, M. J.; Siboni, R. B.; Docter, A. G.; Haley, M. M.; Thornton, C.; Berglund, J. A. ACS Chem. Biol. 2013, 8, 2528-2537.
(4) Warf, M. B.; Nakamori, M.; Matthys, C. M.; Thornton, C. A.; Berglund, J. A. Proc. Nat. Ac. Sci. 2009, 106, 18551–18556.
Coarctate Cyclizations
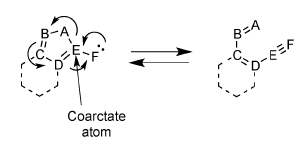
Coarctate reactions can be identified by so-called "coarctations", an atom or linear sequence of atoms in which two bonds are broken and two bonds are formed in a single step. These coarctations are bound by "terminators", which consist of an atom with a lone pair, two atoms complementing the electron shift to a three-membered ring, four atoms complementing the shift to a five-membered ring, etc.
In a paper published by R. Herges 
(DOI:10.1021/ci00017a011) 74 conceivable reactions of the latter type were listed. Some of these reactions were known, most were predicted as possible candidates for future discovery. Our isoindazole cyclization turned out to fit reaction #71 in the list; thus, a new coarctate reaction was found, and explored within our lab for several years as a collaboration with Rainer Herges.
Relevant Publications
(1) Young, B. S.; Herges, R.; Haley, M. M. Chem. Commun. 2012, 48, 9441–9455.
(2) Young, B. S.; Marshall, J. L.; MacDonald, E.; Vonnegut, C. L.; Haley, M. M. Chem. Commun. 2012, 48, 5166–5168.
(3) Young, B. S.; Köhler, F.; Herges, R.; Haley, M. M. J. Org. Chem. 2011, 76, 8483–8487.
(4) McClintock, S. P.; Zakharov, L. N.; Herges, R.; Haley, M. M. Chem. Eur. J. 2011, 17, 6798–6806.
(5) McClintock, S. P.; Shirtcliff, L. D.; Herges, R.; Haley, M. M. J. Org. Chem. 2008, 73, 8755–8762.
(6) Shirtcliff, L. D.; Rivers, J.; Haley, M. M. J. Org. Chem. 2006, 71, 6619–6622.
(7) Shirtcliff, L. D.; Weakley, T. J. R.; Haley, M. M.; Köhler, F.; Herges, R. J. Org. Chem. 2004, 69, 6979–6985.
(8) Kimball, D. B.; Weakley, T. J. R.; Herges, R.; Haley, M. M. J. Am. Chem. Soc. 2002, 124, 13463–13473.
(9) Kimball, D. B.; Herges, R.; Haley, M. M. J. Am. Chem. Soc. 2002, 124, 1572–1573.
(10) Kimball, D. B.; Haley, M. M. Angew. Chem. Int. Ed. 2002, 41, 3338–3351.
(11) Kimball, D. B.; Hayes, A. G.; Haley, M. M. Org. Lett. 2000, 2, 3825–3827.
Aryl-ethynyl Frameworks
Carbon-rich materials are of extreme interest to researchers in many fields, and have become the subject of an increasing number of experimental and theoretical studies due to the isolation and characterization of the fullerenes (C60, C70, etc.) in macroscopic quantities. The major focus of our research into these materials was on two overlapping subsets: (a) carbon networks and models, and (b) molecules with a high C:H ratio. Both studies are based on a class of molecules known as dehydrobenzoannulenes (DBAs).
Calculations predict stable, low energy phases of carbon consisting of stacked, planar carbon layers occupied by sp and sp2 states. The properties of these novel carbon networks are of great relevance in the search for organic conductors, electrochromic display materials, liquid crystals, synthetic ferromagnets, and non-linear optical substances. We have prepared a variety of DBA model compounds for each network in order to compare the chemical and physical properties of the models with those we observe for the corresponding polymeric materials, thus allowing us to probe the monomer/polymer interface.

New synthetic methods developed during the network studies have allowed us to assemble a diverse array of DBA topologies, with nearly 100 macrocycles completed in the past eight years. The structures illustrated below represent some of these molecules, including the first examples of DBAs containing more than two consecutive acetylenic units per side. We also prepared structures incorporating transition metal fragments. Most importantly, the stepwise assembly process used to synthesize our macrocycles allows us to tailor the substituent placement on the aromatic rings, creating for the first time derivatized donor-acceptor structures for nonlinear optical applications.

Relevant Publications
(1) O’Connor, M. J.; Yelle, R. B.; Linz, T. M.; Haley, M. M. Comptes Rendus Chimie 2009, 12, 385–394.
(2) Spitler, E. L.; Haley, M. M. Tetrahedron 2008, 64, 11469–11474.
(3) O’Connor, M. J.; Haley, M. M. Org. Lett. 2008, 10, 3973–3976.
(4) O’Connor, M. J.; Yelle, R. B.; Zakharov, L. N.; Haley, M. M. J. Org. Chem. 2008, 73, 4424–4432.
(5) Samori, S.; Tojo, S.; Fujitsuka, M.; Spitler, E. L.; Haley, M. M.; Majima, T. J. Org. Chem. 2008, 73, 3551–3558.
(6) Spitler, E. L.; Haley, M. M. Org. Biomol. Chem. 2008, 6, 1569–1576.
(7) Spitler, E. L.; Monson, J. M.; Haley, M. M. J. Org. Chem. 2008, 73, 2211–2223.
(8) Tahara, K.; Johnson, C. A.; Fujita, T.; Sonoda, M.; De Schryver, F. C.; De Feyter, S.; Haley, M. M.; Tobe, Y. Langmuir 2007, 23, 10190–10197.
(9) Johnson, C. A.; Lu, Y.; Haley, M. M. Org. Lett. 2007, 9, 3725–3728.
(10) Spitler, E. L.; McClintock, S. P.; Haley, M. M. J. Org. Chem. 2007, 72, 6692–6699.
(11) Samori, S.; Tojo, S.; Fujitsuka, M.; Spitler, E. L.; Haley, M. M.; Majima, T. J. Org. Chem. 2007, 72, 2785–2793.
(12) Spitler, E. L.; Shirtcliff, L. D.; Haley, M. M. J. Org. Chem. 2007, 72, 86–96.
(13) Spitler, E. L.; Johnson, C. A.; Haley, M. M. Chem. Rev. 2006, 106, 5344–5386.
(14) Slepkov, A. D.; Hegmann, F. A.; Tykwinski, R. R.; Kamada, K.; Ohta, K.; Marsden, J. A.; Spitler, E. L.; Miller, J. J.; Haley, M. M. Opt. Lett. 2006, 31, 3315–3317.
(15) Bhaskar, A.; Guda, R.; Haley, M. M.; Goodson J. Am. Chem. Soc. 2006, 128, 13972–13973.
(16) Anand, S.; Varnavski, O.; Marsden, J. A.; Haley, M. M.; Schlegel, H. B.; Goodson, T. J. Phys. Chem. A 2006, 110, 1305–1318.
(17) Hinrichs, H.; Boydston, A. J.; Jones, P. G.; Hess, K.; Herges, R.; Haley, M. M.; Hopf, H. Chem. Eur. J. 2006, 12, 7103–7115.
(18) Marsden, J. A.; Haley, M. M. J. Org. Chem. 2005, 70, 10213–10226.
(19) Hinrichs, H.; Fischer, A. K.; Jones, P. G.; Hopf, H.; Haley, M. M. Org. Lett. 2005, 7, 3793–3795.
(20) Marsden, J. A.; Miller, J. J.; Shirtcliff, L. D.; Haley, M. M. J. Am. Chem. Soc. 2005, 127, 2464–2476.
(21) Johnson, C. A.; Haley, M. M.; Rather, E.; Han, F.; Weakley, T. J. R. Organometallics 2005, 24, 1161–1172.
(22) Marsden, J. A.; O’Conno, M. J.; Haley, M. M. Org. Lett. 2004, 6, 2385–2388.
Metallabenzenes
This area of chemistry pushes the frontiers of our understanding of the bonding and reactivity in organometallics as often time systems of this type defy conventional wisdom. The key to our research is the preparation of cyclopropenes containing additional unsaturated moieties and their subsequent reaction with transition-metal reagents to yield novel organometallic complexes.
A metallabenzene is a transition-metal analog of benzene in which one methine (CH) is replaced by an isoelectronic MLn fragment, yet the molecule retains "aromatic" physical and chemical properties. Although two dozen or so such structures are known, there was no general route that allowed entry into this class of molecules. Suitably substituted 3-vinylcyclopropenes made a general route accessible due to the inherent reactivity of the strained cyclopropene to undergo ring cleavage or ring expansion. Depending upon the substitution pattern on the cyclopropene and the organometallic reagent used, it became possible to isolate the corresponding metallabenzene valence isomers.

Using Vaska complexes and a 1,2-diphenyl cyclopropene derivative, we have recently prepared several new "iridabenzenes" as well as the first examples of "iridabenzvalenes" (Figure 1). Like a normal benzene valence isomer, iridabenzvalene cleanly rearranges to an iridabenzene upon heating.
 |
 |
Figure 1. Molecular structures of iridabenzene and iridabenzvalene, respectively; ellipsoids drawn at the 30% level.
We have recently extended this new methodology for metallabenzenes to utilize other metals. The formation of a platinabenzene and a rhodabenzvalene, which are shown in Figure 2, illustrate the diversity of our route.
 |
 |
Figure 2. Molecular structures of a platinabenzene (left, 2a) and a rhodabenzvalene (right, 2b).
Relevant Publications
(1) Jacob, V.; Landorf, C. W.; Zakharov, L. N.; Weakley, T. J. R.; Haley, M. M. Organometallics 2009, 28, 5183–5190.
(2) Wu, H.-P.; Ess, D. H.; Lanza, S.; Weakley, T. J. R.; Houk, K. N.; Baldridge, K. K.; Haley, M. M. Organometallics 2007, 26, 3957–3968.
(3) Johnson II, C. A.; Baker, B. A.; Berryman, O. B.; Zakharov, L. N.; O’Connor, M. J.; Haley, M. M. J. Organomet. Chem. 2006, 691, 413–421.
(4) Landorf, C. W.; Haley, M. M. Ang. Chem. Int. Ed. 2006, 45, 3914–3936.
(5) Wu, H.-P.; Weakley, T. J. R.; Haley, M. M. Chem. Eur. J. 2005, 11, 1191–1200.
