Indenofluorene Scaffolds
Polycyclic hydrocarbons that possess extended π-conjugation are of tremendous interest due to their potential use in optical and
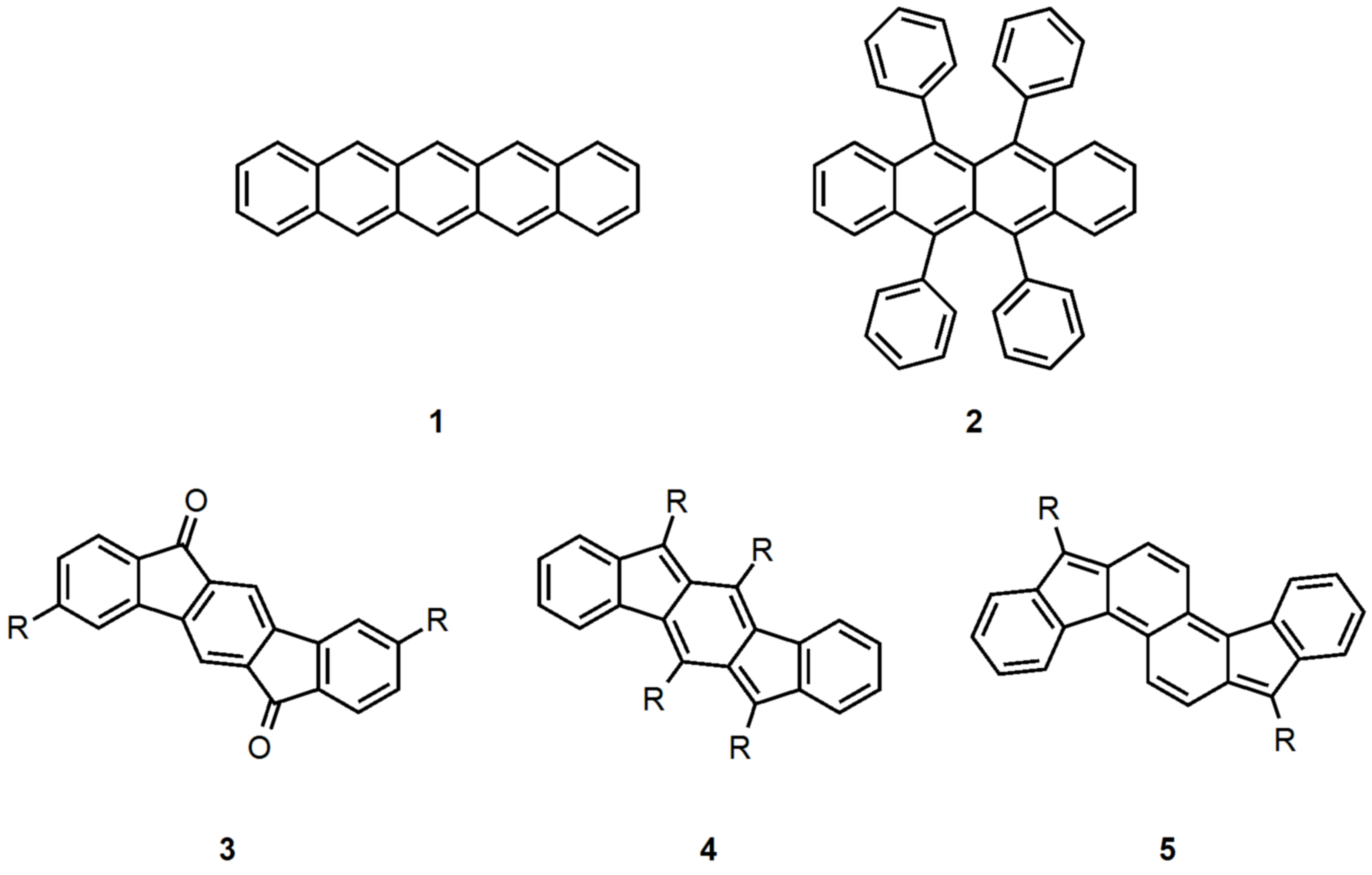 electronic device applications. While a majority of studies have focused on acenes and their derivatives (for example, compounds 1-2), these
systems are susceptible to degradation due to environmental effects, through oxidative or photolytic pathways; thus, there is a pressing need for
alternative, acene-like topologies. Current research in the lab is focused on molecules based on or inspired by the indenofluorene (IF) skeleton,
such as compounds 3-5. Over the last few years, we have adapted and/or developed general methods for the assembly of a variety of fully conjugated
IF derivatives and initiated exploration of their materials properties. We have shown that IFs can be prepared in gram quantities with good overall
yields and excellent purity using methodologies amenable to large-scale production.
electronic device applications. While a majority of studies have focused on acenes and their derivatives (for example, compounds 1-2), these
systems are susceptible to degradation due to environmental effects, through oxidative or photolytic pathways; thus, there is a pressing need for
alternative, acene-like topologies. Current research in the lab is focused on molecules based on or inspired by the indenofluorene (IF) skeleton,
such as compounds 3-5. Over the last few years, we have adapted and/or developed general methods for the assembly of a variety of fully conjugated
IF derivatives and initiated exploration of their materials properties. We have shown that IFs can be prepared in gram quantities with good overall
yields and excellent purity using methodologies amenable to large-scale production.
In addition to being challenging synthetic targets, IFs help to provide answers to fundamental questions about structure, bonding, and reactivity in expanded, conjugated structures. They also have the potential to act as rigid, planar, electron-accepting cores for the formation of advanced materials with novel electronic properties. We are exploiting the materials potential of IFs via a combined experimental and theoretical approach, with an emphasis toward the use of indenofluorenes as organic semiconductors in devices. Importantly, we demonstrated recently that single crystals of an aryl-substituted IF could serve as an active layer in an organic field-effect transistor that exhibits ambipolar behavior.
Selected Publications
Synthesis and Characterization of Two Unsymmetrical Indenofluorene Analogues: Benzo[5,6]-s-indaceno[1,2-b]thiophene and Benzo[5,6]-s-indaceno[2,1-b]thiophene

Marshall. J. L.; O'Neal, N. J.; Zakharov, L. N.; Haley, M. M. J. Org. Chem. 2016, in press.
DOI: 10.1021/10.1021/acs.joc.6b00340
Synthesis of Open-Shell Ladder π-Systems by Catalytic C–H Annulation of Diarylacetylenes

Maekawa, T.; Ueno, H.; Segawa, Y.; Haley, M. M.; Itami, K. Chem. Sci. 2016, 7, 650-654.
DOI: 10.1039/C5SC03391H
Synthesis and properties of fully conjugated indacenediselenophene and diindenoselenophene derivatives

Marshall. J. L.; Rudebusch, G. E.; Vonnegut, C. L.; Zakharov, L. N.; Haley, M. M. Tetrahedron Lett. 2015, 56, 3235-3239.
DOI: 10.1016/j.tetlet.2014.12.096
Synthesis and Optoelectronic Properties of Indeno[1,2-b]fluorene-6,12-dione Donor–Acceptor–Donor Triads

Frederickson, C. K.; Haley, M. M. J. Org. Chem. 2014, 79, 11241-11245.
DOI: 10.1021/jo502009p
Unusually short excited state lifetimes of indenofluorene and fluorenofluorene derivatives result from a conical intersection

Rose, B. D.; Shoerb, L. E.; Wasielewski, M. R.; Haley, M. M. Chem. Phys. Lett. 2014, 616-617, 137-141.
DOI: 10.1016/j.cplett.2014.10.031
Scalable synthesis of 5,11-diethynylated indeno[1,2-b]fluorene-6,12-diones and exploration of their solid state packing

Rose, B. D.; Santa Maria, P. J.; Fix, A.G.; Vonnegut, C. L.; Zakharov, L.; Parkin, S. R.; Haley, M. M. Beilstein J. Org. Chem. 2014, 10, 2122–2130.
DOI: 10.3762/bjoc.10.219
Quinoidal diindenothienoacenes: Synthesis and properties of new functional organic materials

Rudebusch, G. E.; Fix, A. G.; Henthorn, H. A.; Vonnegut, C. L.; Zakharov, L.; Haley, M. M. Chem. Sci. 2014, 5, 3627-3633.
DOI: 10.1039/C4SC01432D
Experimental and Computational Studies of the Neutral and Reduced States of Indeno[1,2-b]fluorene

Rose, B. D.; Sumner, N. J.; Filatov, A. S.; Peters, S. J.; Zakharov, L. N.; Petrukhina, M. A.; Haley, M. M. J. Am. Chem. Soc. 2014, 136, 9181–9189.
DOI: 10.1021/ja503870z
Synthesis and properties of fully-conjugated indacenedithiophenes

Young, B. S.; Chase, D. T.; Marshall, J. L.; Vonnegut, C. L.; Zakharov, L. N.; Haley, M. M. Chem. Sci. 2014, 5, 1008-1014.
DOI: 10.1039/C3SC53181C
Indeno[2,1-c]fluorene: A New Electron-Accepting Scaffold for Organic Electronics

Fix, A. G.; Deal, P. E.; Vonnegut, C. L.; Rose, B. D.; Zakharov, L. N.; Haley, M. M. Org. Lett. 2013, 15, 1362–1365.
DOI: 10.1021/ol400318z
6,12-Diarylindeno[1,2-b]fluorenes: Syntheses, Photophysics, and Ambipolar OFETs

Chase, D. T.; Fix, A. G.; Kang, S. J.; Rose, B. D.; Weber, C. D.; Zhong, Y.; Zakharov, L. N.; Lonergan, M. C.; Nuckolls, C.; Haley, M. M. J. Am. Chem. Soc. 2012, 134, 10349–10352.
DOI: 10.1021/ja303402p
Fluoreno[4,3-c]fluorene: A Closed-Shell, Fully Conjugated Hydrocarbon

Rose, B. D.; Vonnegut, C. L.; Zakharov, L. N.; Haley, M. M. Org. Lett. 2012, 14, 2426–2429.
DOI: 10.1021/ol300942z
Electron-Accepting 6,12-Diethynylindeno[1,2-b]fluorenes: Synthesis, Crystal Structures, and Photophysical Properties

Chase, D. T.; Fix, A. G.; Rose, B. D.; Weber, C. D.; Nobusue, S.; Stockwell, C. E.; Zakharov, L. N.; Lonergan, M. C.; Haley, M. M. Angew. Chem. Int. Ed. 2011, 50, 11103–11106.
DOI: 10.1002/anie.201104797
Synthesis, Crystal Structures, and Photophysical Properties of Electron-Accepting Diethynylindenofluorenediones

Rose, B. D.; Chase, D. T.; Weber, C. D.; Zakharov, L. N.; Lonergan, M. C.; Haley, M. M. Org. Lett. 2011, 13, 2106–2109.
DOI: 10.1021/ol200525g
Indeno[1,2-b]fluorenes: Fully Conjugated Antiaromatic Analogues of Acenes

Chase, D. T.; Rose, B. D.; McClintock, S. P.; Zakharov, L. N.; Haley, M. M. Angew. Chem. Int. Ed. 2011, 50, 1127–1130.
DOI: 10.1002/anie.201006312
