



Russell J. Donnelly
541-346-4226 (Tel)
541-346-5861 (Fax)
![]()
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |
![]()
![]() The
Observed Properties of Liquid Helium
The
Observed Properties of Liquid Helium
![]() at the Saturated Vapor
Pressure
at the Saturated Vapor
Pressure
Chapter
16. Latent Heat of Vaporization
Adopted Database
Author(s) |
Key # |
Range(Å-1) |
| Van Dijk and Durieux | 1 | 1
|
| Berman and Poulter | 2 | 1.5
|
| Dana and Onnes | 3 | 2.2
|
| Ter Harmsel et al. | 4 | 2.2
|
| Theory | 5 | 0
|
Comments and Key to Authors
1)
Ref. 3. Discussion of data from references 1 - 3.
2) Ref. 2. Uncertainties:![]() 0.1%
.
0.1%
.
3) Ref. 1. Pioneering qualitative measurements.
4) Ref. 4. Uncertainties: Random ![]() 0.1%
, systematic
0.1%
, systematic ![]() 0.03%.
0.03%.
5) The latent heat at absolute zero is taken as Lo = 59.83 J/mol. At low
temperatures, L=Lo+(5/2)RT which was used to 0.9 K. Here, R
= 8.31451 J/mol-K.
.
Table
16.1. Adopted database for latent heat of vaporization of liquid 4He.
T90 (K) |
L (J/mol) |
Key |
T90 (K) |
L (J/mol) |
Key |
| 0.0000 | 59.83 | 5 | 2.4049 | 91.91 | 2 |
| 0.1000 | 61.91 | 5 | 2.4049 | 92.50 | 3 |
| 0.2000 | 63.99 | 5 | 2.4049 | 91.94 | 4 |
| 0.3000 | 66.07 | 5 | 2.5052 | 92.92 | 1 |
| 0.4000 | 68.14 | 5 | 2.5052 | 92.46 | 2 |
| 0.5000 | 70.22 | 5 | 2.5052 | 92.45 | 4 |
| 0.6000 | 72.30 | 5 | 2.6054 | 92.98 | 4 |
| 0.7000 | 74.38 | 5 | 2.6054 | 92.80 | 1 |
| 0.8000 | 76.46 | 5 | 2.6054 | 92.95 | 2 |
| 0.9000 | 78.54 | 5 | 2.6054 | 93.40 | 3 |
| 1.0028 | 80.22 | 1 | 2.7057 | 93.25 | 1 |
| 1.1030 | 82.22 | 1 | 2.7057 | 93.31 | 2 |
| 1.2033 | 84.17 | 1 | 2.7057 | 93.50 | 4 |
| 1.3033 | 86.03 | 1 | 2.8060 | 93.58 | 1 |
| 1.4035 | 87.76 | 1 | 2.8060 | 93.59 | 2 |
| 1.5036 | 89.36 | 1 | 2.8060 | 94.20 | 3 |
| 1.5036 | 89.70 | 2 | 2.8060 | 93.89 | 4 |
| 1.6037 | 90.74 | 1 | 2.9061 | 93.81 | 1 |
| 1.6037 | 90.86 | 2 | 2.9061 | 93.81 | 2 |
| 1.7039 | 91.88 | 1 | 2.9061 | 94.16 | 4 |
| 1.7039 | 91.92 | 2 | 3.0063 | 93.91 | 1 |
| 1.8041 | 92.72 | 1 | 3.0063 | 93.90 | 2 |
| 1.8041 | 92.72 | 2 | 3.0063 | 94.70 | 3 |
| 1.9042 | 93.17 | 1 | 3.0063 | 94.29 | 4 |
| 1.9042 | 93.13 | 2 | 3.1064 | 93.90 | 1 |
| 2.0042 | 93.13 | 1 | 3.1064 | 93.90 | 2 |
| 2.0042 | 93.01 | 2 | 3.1064 | 94.30 | 4 |
| 2.1044 | 92.32 | 1 | 3.2066 | 93.75 | 1 |
| 2.1044 | 92.03 | 2 | 3.2066 | 93.78 | 2 |
| 2.1546 | 91.47 | 1 | 3.2066 | 94.60 | 3 |
| 2.1546 | 91.16 | 2 | 3.2066 | 94.19 | 4 |
| 2.1646 | 91.21 | 1 | 3.3067 | 93.44 | 1 |
| 2.1646 | 90.98 | 2 | 3.3067 | 93.50 | 2 |
| 2.1748 | 90.86 | 1 | 3.3067 | 93.95 | 4 |
| 2.1748 | 90.79 | 2 | 3.4069 | 92.99 | 1 |
| 2.1946 | 90.75 | 1 | 3.4069 | 93.06 | 2 |
| 2.1946 | 90.71 | 2 | 3.4069 | 93.90 | 3 |
| 2.2046 | 90.75 | 1 | 3.4069 | 93.56 | 4 |
| 2.2046 | 90.77 | 2 | 3.5070 | 92.42 | 1 |
| 2.2046 | 91.40 | 3 | 3.5070 | 92.46 | 2 |
| 2.2046 | 91.07 | 4 | 3.5070 | 92.99 | 4 |
| 2.2546 | 90.91 | 1 | 3.6070 | 91.64 | 1 |
| 2.2546 | 91.05 | 2 | 3.6070 | 91.67 | 2 |
| 2.3047 | 91.16 | 1 | 3.6070 | 92.70 | 3 |
| 2.3047 | 91.34 | 2 | 3.6070 | 92.27 | 4 |
| 2.3047 | 91.51 | 4 | 3.7071 | 90.71 | 1 |
| 2.4049 | 91.73 | 1 | 3.7071 | 90.71 | 2 |
![]()
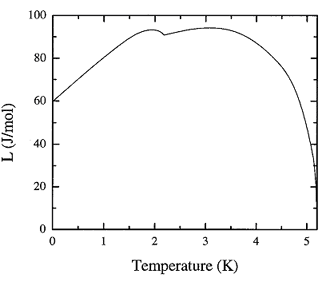
Figure 16.1. The recommended values for the latent heat of vaporization of liquid 4He as a function of temperature at the saturated vapor pressure.
Table 16.2. Knots and coefficients for the spline fit of the latent heat of vaporization of liquid 4He.
Knots |
Coefficients |
| K(1) = 0.000000 | C(1) = 59.82983 |
| K(2) = 0.000000 | C(2) = 68.59642 |
| K(3) = 0.000000 | C(3) = 82.78576 |
| K(4) = 0.000000 | C(4) = 94.70215 |
| K(5) = 1.268000 | C(5) = 92.50350 |
| K(6) = 1.980630 | C(6) = 90.73236 |
| K(7) = 2.176800 | C(7) = 93.68727 |
| K(8) = 2.176800 | C(8) = 97.81899 |
| K(9) = 2.176800 | C(9) = 80.05877 |
| K(10) = 3.726500 | C(10) = 64.58454 |
| K(11) = 4.378900 | C(11) = 29.47641 |
| K(12) = 5.106127 | C(12) = 15.94783 |
| K(13) = 5.178168 | C(13) = 3.110047E-04 |
| K(14) = 5.195767 | |
| K(15) = 5.195767 | |
| K(16) = 5.195767 | |
| K(17) = 5.195767 |
![]()
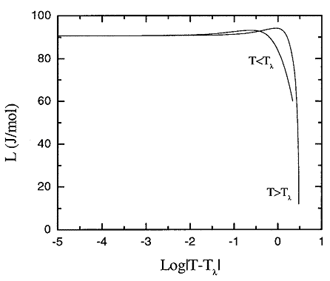
Figure 16.2. Detail of the recommended values latent heat of vaporization about the lambda transition.
Table 16.3. Recommended values of the latent heat of vaporization of liquid 4He as a function of temperature at the saturated vapor pressure.
T90 (K) |
L (J/mol) |
T90 (K) |
L (J/mol) |
| 0.00 | 59.83 | 2.35 | 91.71 |
| 0.05 | 60.87 | 2.40 | 91.98 |
| 0.10 | 61.91 | 2.45 | 92.24 |
| 0.15 | 62.95 | 2.50 | 92.50 |
| 0.20 | 64.00 | 2.55 | 92.74 |
| 0.25 | 65.04 | 2.60 | 92.97 |
| 0.30 | 66.08 | 2.65 | 93.18 |
| 0.35 | 67.13 | 2.70 | 93.38 |
| 0.40 | 68.17 | 2.75 | 93.56 |
| 0.45 | 69.21 | 2.80 | 93.71 |
| 0.50 | 70.24 | 2.85 | 93.85 |
| 0.55 | 71.28 | 2.90 | 93.96 |
| 0.60 | 72.31 | 2.95 | 94.05 |
| 0.65 | 73.33 | 3.00 | 94.11 |
| 0.70 | 74.35 | 3.05 | 94.14 |
| 0.75 | 75.37 | 3.10 | 94.14 |
| 0.80 | 76.38 | 3.15 | 94.11 |
| 0.85 | 77.38 | 3.20 | 94.05 |
| 0.90 | 78.37 | 3.25 | 93.94 |
| 0.95 | 79.36 | 3.30 | 93.80 |
| 1.00 | 80.33 | 3.35 | 93.63 |
| 1.05 | 81.30 | 3.40 | 93.41 |
| 1.10 | 82.26 | 3.45 | 93.14 |
| 1.15 | 83.21 | 3.50 | 92.84 |
| 1.20 | 84.14 | 3.55 | 92.48 |
| 1.25 | 85.06 | 3.60 | 92.08 |
| 1.30 | 85.97 | 3.65 | 91.63 |
| 1.35 | 86.87 | 3.70 | 91.13 |
| 1.40 | 87.73 | 3.75 | 90.58 |
| 1.45 | 88.56 | 3.80 | 89.97 |
| 1.50 | 89.35 | 3.85 | 89.31 |
| 1.55 | 90.09 | 3.90 | 88.59 |
| 1.60 | 90.77 | 3.95 | 87.82 |
| 1.65 | 91.38 | 4.00 | 87.00 |
| 1.70 | 91.91 | 4.05 | 86.13 |
| 1.75 | 92.36 | 4.10 | 85.20 |
| 1.80 | 92.72 | 4.15 | 84.22 |
| 1.85 | 92.98 | 4.20 | 83.19 |
| 1.90 | 93.13 | 4.25 | 82.11 |
| 1.95 | 93.16 | 4.30 | 80.98 |
| 2.00 | 93.07 | 4.35 | 79.80 |
| 2.05 | 92.80 | 4.40 | 78.57 |
| 2.10 | 92.27 | 4.45 | 77.27 |
| 2.18 | 90.75 | 4.50 | 75.86 |
| 2.18 | 90.75 | 4.55 | 74.32 |
| 2.18 | 90.75 | 4.60 | 72.59 |
| 2.18 | 90.75 | 4.65 | 70.64 |
| 2.18 | 90.74 | 4.70 | 68.44 |
| 2.18 | 90.74 | 4.75 | 65.94 |
| 2.18 | 90.74 | 4.80 | 63.11 |
| 2.18 | 90.74 | 4.85 | 59.90 |
| 2.18 | 90.73 | 4.90 | 56.28 |
| 2.18 | 90.73 | 4.95 | 52.22 |
| 2.18 | 90.73 | 5.00 | 47.67 |
| 2.20 | 90.87 | 5.05 | 42.59 |
| 2.25 | 91.15 | 5.10 | 36.95 |
| 2.30 | 91.43 | 5.15 | 29.34 |
![]()
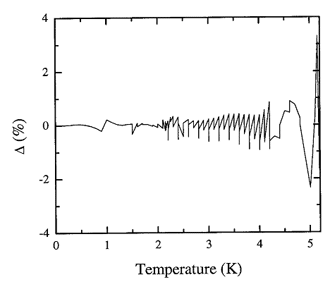
Figure 16.3. The fractional deviation of values of the adopted database from the recommended values for the latent heat expressed in percent.
Chronological Bibliography for Mutual Friction
1 |
L. I. Dana and K. H. Onnes, "Further Experiments with Liquid Helium. B. A. Preliminary Determinations of the Latent Heat of Vaporization of Liquid Helium," Proc. Roy. Acad. Amsterdam 29, 1051-1061 (1926). |
| 2 | R. Berman and J. Poulter, “On the Latent Heat and Vapour Density of Helium,” Phil. Mag. 43, 1047-1054 (1952). |
| 3 | H. Van Dijk and M. Durieux, “The Temperature Scale in the Liquid Helium Region,” in Progress in Low Temperature Physics, edited by C. J. Gorter (North Holland, 1957), Vol. 2, pp. 431-464. |
| 4 | H. Ter Harmsel, H. Van Dijk, and M. Durieux, “The Heat of Vaporization of Helium,” Physica 36, 620-636 (1967). |
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |

