



Russell J. Donnelly
541-346-4226 (Tel)
541-346-5861 (Fax)
![]()
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |
![]() The
Observed Properties of Liquid Helium
The
Observed Properties of Liquid Helium
![]() at the Saturated Vapor
Pressure
at the Saturated Vapor
Pressure
Chapter
1. Density, Thermal Expansion, and Dielectric Constant
The
density of liquid 4He at saturated vapor pressure (SVP) has been measured
by a number of authors (see the chronological bibliography at the end
of this chapter). We have chosen as our source the recent work of Niemela
and Donnelly 40, who measured the dielectric constant of liquid helium
and resolved many of the discrepancies which existed in the older literature.
Dielectric constants were converted to density using the Clausius-Mossotti
relation
![]()
![]()
where
M = 4.0026 g/mol is the molecular weight of helium and is the
molar polarizability. They adopted the value ![]() M
=0.123296 cm3/mol deduced by Harris-Lowe and Smee30 from their
measurements of dielectric constant and Kerr and Taylor's18 measurement
of the density.
M
=0.123296 cm3/mol deduced by Harris-Lowe and Smee30 from their
measurements of dielectric constant and Kerr and Taylor's18 measurement
of the density.
Although Niemela and Donnelly did not make any measurements below the
density minimum, they provided a continuous representation of the density
and expansion coefficient to zero temperature using theoretical methods
described in detail in their paper obtaining values of the density which
are described by the following equation up to 1.344K
![]()
where . The coefficients of Eq.(2) are given in Table 1.1. The authors fit the reduced density
![]()
![]()
to a function of the form:
![]()
![]()
where
t=T-T![]() .
Here T
.
Here T![]() =2.1768k , and P
=2.1768k , and P![]() =0.1461087
g/cm3 . Because they wished to tabulate the expansion coefficient
over the entire range of temperatures, it was desirable that not only
the density, but also its first derivative should smoothly join to the
calculated values given by Eq.(1.2). This was best achieved by using Eq.(1.2)
up to 1.344K and using Eq.(1.4) for data in the ranges 1.334k
=0.1461087
g/cm3 . Because they wished to tabulate the expansion coefficient
over the entire range of temperatures, it was desirable that not only
the density, but also its first derivative should smoothly join to the
calculated values given by Eq.(1.2). This was best achieved by using Eq.(1.2)
up to 1.344K and using Eq.(1.4) for data in the ranges 1.334k ![]() T
T ![]() T
T![]() and T
and T![]() <
T < 4.9k. The resulting set of equations and their derivatives
provide a continuous representation of the density and expansion coefficient
from near 0 K to 4.9 K. The coefficients for Eq.(1.4) are given in Table
1.1. The mean fractional deviation of the density data from the fit is
0.5 x10-6. The density is tabulated in g/cm3. Multiply
the entries by 1000 to convert to kg/m3.
<
T < 4.9k. The resulting set of equations and their derivatives
provide a continuous representation of the density and expansion coefficient
from near 0 K to 4.9 K. The coefficients for Eq.(1.4) are given in Table
1.1. The mean fractional deviation of the density data from the fit is
0.5 x10-6. The density is tabulated in g/cm3. Multiply
the entries by 1000 to convert to kg/m3.
Table 1.1. Coefficients for Eqs.(2), (4), (5) and (6).
| i | ai
X 103 |
bi X 103 | mi X 103 | si X 103 | ||
1.344K-T |
T |
1.344K-T |
T |
0-1.344 K |
0-1.344 k |
|
| 1 | -7.57537 | -7.94605 | 3.79937 | -30.3511 | -1.26935 | -0.117818 |
| 2 | 6.87483 | 5.07051 | 1.86557 | -10.2326 | 7.12413 | 1.64045 |
| 3 | 4.88345 | -3.00636 | -16.7461 | -6.18750 | ||
| 4 | 0 | 0.240720 | 8.75342 | 13.4293 | ||
| 5 | 0 | -2.45749 | -11.3971 | |||
| 6 | 0 | 1.53454 | 2.94176 | |||
| 7 | 0 | -0.308182 | ||||
In Figure 1.1 we plot Eqs.
1.2 and 1.4 for the density. The following expressions give the deduced
thermal expansion coefficient: For 0.15 ![]() T
T ![]() 1.344 :
1.344 :
![]()
![]()
For
1.33 ![]() T<
T
T<
T![]() and T
and T![]() < T < 4.9
< T < 4.9
![]()
![]()
The
coefficients are listed in Table 1.1. The method of obtaining Eq. (1.5)
is explained by Niemela and Donnelly. Notice that the average fractional
deviation of Eq.(1.5) from the theoretical values on which it is based
is 1x10-6, so that its valid temperature range begins 150 mK
above absolute zero. Eq.(1.6) was derived from Eq.(1.4). Also be aware
that this data was not taken in great temperature detail near the lambda
point.
Figure 1.2 shows the expansion coefficient over the entire range of temperatures.
The dielectric constant can be obtained as follows:
![]()
![]()
![]()
![]()
This roundabout method of getting the dielectric constant arises because Niemela and Donnelly fitted the density (their main goal) after converting individual dielectric constant measurements to density by means of Eq.(1.1).
![]()
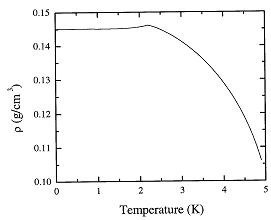
Fig. 1.1. The recommended values of the density of liquid 4He as a function of temperature at the saturated vapor pressure.
![]()
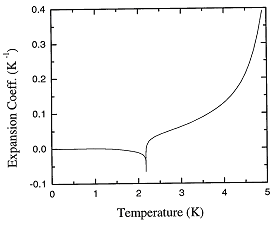
Figure 1.2. The recommended values of the thermal expansion coefficient of liquid 4He at the saturated vapor pressure as a function of temperature.
![]()
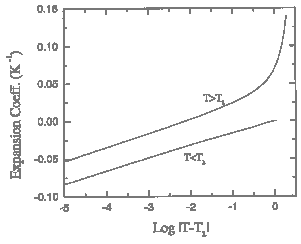
Figure 1.3. Detail of the recommended values for the thermal expansion coefficient of liquid 4He near the lambda transition.
![]()
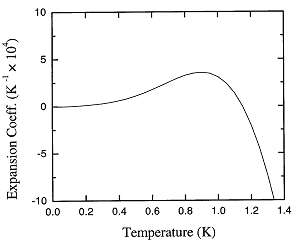
Figure 1.4. The recommended values of the thermal expansion coefficient of liquid 4He at low temperatures.
Table 1.2. Recommended values of the dielectric constant, density and thermal expansion coefficient of liquid 4He at saturated vapor pressure.
T90(K) |
p(g/cm3) |
103 |
T90(K) |
p(g/cm3) |
103 |
||
| 0.00 | 1.057255 | 1.451397E-1 | 0.000 | 2.50 | 1.057135 | 1.448402E-1 | 39.4 |
| 0.10 | 1.057255 | 1.451397E-1 | 0.001 | 2.55 | 1.057017 | 1.445467E-1 | 41.8 |
| 0.15 | 1.057255 | .451396E-1 | 10.004 | 2.60 | 1.056892 | 1.442368E-1 | 44.1 |
| 0.20 | 1.057255 | 1.451395E-1 | 0.011 | 2.65 | 1.056761 | 1.439114E-1 | 46.3 |
| 0.25 | 1.057255 | 1.451395E-1 | 0.018 | 2.70 | 1.056625 | 1.435712E-1 | 48.4 |
| 0.30 | 1.057255 | 1.451393E-1 | 0.028 | 2.75 | 1.056482 | 1.432164E-1 | 50.6 |
| 0.35 | 1.057255 | 1.451391E-1 | 0.042 | 2.80 | 1.056334 | 1.428472E-1 | 52.7 |
| 0.40 | 1.057255 | 1.451388E-1 | 0.058 | 2.85 | 1.056180 | 1.424638E-1 | 54.8 |
| 0.45 | 1.057254 | 1.451384E-1 | 0.080 | 2.90 | 1.056020 | 1.420661E-1 | 57.0 |
| 0.50 | 1.057254 | 1.451377E-1 | 0.107 | 2.95 | 1.055854 | 1.416538E-1 | 59.2 |
| 0.55 | 1.057254 | 1.451368E-1 | 0.139 | 3.00 | 1.055683 | 1.412269E-1 | 61.5 |
| 0.60 | 1.057253 | 1.451356E-1 | 0.175 | 3.05 | 1.055505 | 1.407850E-1 | 63.9 |
| 0.65 | 1.057253 | 1.451342E-1 | 0.214 | 3.10 | 1.055322 | 1.403279E-1 | 66.3 |
| 0.70 | 1.057252 | 1.451324E-1 | 0.254 | 3.15 | 1.055132 | 1.398551E-1 | 68.7 |
| 0.75 | 1.057251 | 1.451304E-1 | 0.292 | 3.20 | 1.054936 | 1.393663E-1 | 71.3 |
| 0.80 | 1.057250 | 1.451281E-1 | 0.325 | 3.25 | 1.054733 | 1.388611E-1 | 74.0 |
| 0.85 | 1.057249 | 1.451257E-1 | 0.348 | 3.30 | 1.054523 | 1.383390E-1 | 76.7 |
| 0.90 | 1.057248 | 1.451232E-1 | 0.357 | 3.35 | 1.054307 | 1.377997E-1 | 79.5 |
| 0.95 | 1.057247 | 1.451207E-1 | 0.345 | 3.40 | 1.054084 | 1.372427E-1 | 82.5 |
| 1.00 | 1.057246 | 1.451183E-1 | 0.309 | 3.45 | 1.053853 | 1.366675E-1 | 85.5 |
| 1.05 | 1.057246 | 1.451163E-1 | 0.242 | 3.50 | 1.053615 | 1.360736E-1 | 88.7 |
| 1.10 | 1.057245 | 1.451150E-1 | 0.138 | 3.55 | 1.053369 | 1.354605E-1 | 92.0 |
| 1.15 | 1.057245 | 1.451144E-1 |
-0.008 | 3.60 | 1.053115 | 1.348278E-1 | 95.3 |
| 1.20 | 1.057245 | 1.451151E-1 | -0.200 | 3.65 | 1.052853 | 1.341748E-1 | 98.9 |
| 1.25 | 1.057246 | 1.451173E-1 | -0.442 | 3.70 | 1.052583 | 1.335009E-1 | 103 |
| 1.30 | 1.057248 | 1.451215E-1 | -0.737 | 3.75 | 1.052305 | 1.328054E-1 | 106 |
| 1.35 | 1.057250 | 1.451281E-1 | -1.08 | 3.80 | 1.052017 | 1.320877E-1 | 110 |
| 1.40 | 1.057254 | 1.451373E-1 | -1.45 | 3.85 | 1.051720 | 1.313467E-1 | 115 |
| 1.45 | 1.057259 | 1.451493E-1 | -1.87 | 3.90 | 1.051414 | 1.305817E-1 | 119 |
| 1.50 | 1.057265 | 1.451646E-1 | -2.36 | 3.95 | 1.051097 | 1.297914E-1 | 124 |
| 1.55 | 1.057273 | 1.451837E-1 | -2.91 | 4.00 | 1.050770 | 1.289745E-1 | 129 |
| 1.60 | 1.057282 | 1.452071E-1 | -3.53 | 4.05 | 1.050432 | 1.281296E-1 | 134 |
| 1.65 | 1.057293 | 1.452352E-1 | -4.23 | 4.10 | 1.050082 | 1.272549E-1 | 140 |
| 1.70 | 1.057307 | 1.452686E-1 | -4.99 | 4.15 | 1.049719 | 1.263483E-1 | 146 |
| 1.75 | 1.057323 | 1.453079E-1 | -5.84 | 4.20 | 1.049343 | 1.254075E-1 | 153 |
| 1.80 | 1.057341 | 1.453538E-1 | -6.79 | 4.25 | 1.048952 | 1.244297E-1 | 160 |
| 1.85 | 1.057362 | 1.454070E-1 | -7.86 | 4.30 | 1.048545 | 1.234117E-1 | 168 |
| 1.90 | 1.057387 | 1.454684E-1 | -9.07 | 4.35 | 1.048121 | 1.223498E-1 | 177 |
| 1.95 | 1.057416 | 1.455394E-1 | -10.5 | 4.40 | 1.047677 | 1.212398E-1 | 187 |
| 2.00 | 1.057449 | 1.456217E-1 | -12.2 | 4.45 | 1.047213 | 1.200768E-1 | 198 |
| 2.05 | 1.057488 | 1.457181E-1 | -14.4 | 4.50 | 1.046725 | 1.188552E-1 | 211 |
| 2.10 | 1.057534 | 1.458340E-1 | -17.7 | 4.55 | 1.046211 | 1.175686E-1 | 225 |
| 2.15 | 1.057594 | 1.459840E-1 | -24.7 | 4.60 | 1.045669 | 1.162098E-1 | 241 |
| 2.20 | 1.057643 | 1.461049E-1 | 9.64 | 4.65 | 1.045095 | 1.147706E-1 | 258 |
| 2.25 | 1.057596 | 1.459877E-1 | 20.7 | 4.70 | 1.044485 | 1.132419E-1 | 279 |
| 2.30 | 1.057526 | 1.458148E-1 | 26.4 | 4.75 | 1.043836 | 1.116131E-1 | 301 |
| 2.35 | 1.057443 | 1.456071E-1 | 30.5 | 4.80 | 1.043143 | 1.098727E-1 | 328 |
| 2.40 | 1.057349 | 1.453727E-1 | 33.8 | 4.85 | 1.042400 | 1.080076E-1 | 358 |
| 2.45 | 1.057246 | 1.451162E-1 | 36.7 | 4.90 | 1.041602 | 1.060033E-1 | 392 |
1.1 Chronological Bibliography for Density and Expansion Coefficient
1 |
K. H. Onnes, "The Liquefaction of Helium," Leiden Comm. 108, 3-23 (1908). |
| 2 | K. H. Onnes, “Further Experiments with Liquid Helium,” Proc. Roy. Acad., Amsterdam 170b, 1093-1119 (1911). |
| 3 | K. H. Onnes and J. D. A. Boks, “Further Experiments with Liquid Helium,” Leiden Comm. 170b, 18-23 (1924). |
| 4 | M. Wolfke and K. H. Onnes, “Further Experiments in Liquid Helium. V. On the Dielectric Constant of Liquid Helium,” Proc. Roy. Acad., Amsterdam 27, 621-626 (1924). |
| 5 | E. Mathias, C. A. Crommelin, K. H. Onnes, and J. C. Swallow, “The Rectilinear Diameter of Helium,” Proc. Roy. Acad., Amsterdam 28, 526-528 (1925). |
| 6 | M. Wolfke and W. H. Keesom, “On the Change of the Dielectric Constant of Liquid Helium with the Temperature. Provisional Measurements,” Proc. Roy. Acad., Amsterdam 31, 81-94 (1928). |
| 7 | W. H. Keesom and A. P. Keesom, “Isopycnals of Liquid Helium,” Proc. Roy. Acad., Amsterdam 36, 482-487 (1933). |
| 8 | W. H. Keesom and A. P. Keesom, “Isopycnals of Liquid Helium II,” Proc. Roy. Acad., Amsterdam 36, 612-615 (1933). |
| 9 | H. E. Johns and J. O. Wilhelm, “The Refractive Indices of Liquid Helium I and Helium II,” Can. J. Res. A16, 131-137 (1938). |
| 10 | C. J. Grebenkamper and J. P. Hagen, “The Dielectric Constant of Liquid Helium,” Phys. Rev. 80, 89-91 (1950). |
| 11 | K. R. Atkins and M. H. Edwards, “Coefficients of Expansion of Liquid Helium,” Phys. Rev. 97, 1429-1432 (1955). |
| 12 | E. C. Kerr, “Density of Liquid 4He,” J. Chem. Phys. 26, 511-514 (1957). |
| 13 | F. J. Edeskuty and R. H. Sherman, “P-V-T Relations of Liquid 3He and 4He,” in 5th International Conference on Low Temperature Physics and Chemistry, edited by J. R. Dillinger (University of Wisconsin, Madison, 1958), pp. 102-106. |
| 14 | M. H. Edwards, “Refractive Index of 4He Liquid,” Can. J. Phys. 36, 884-898 (1958). |
| 15 | E. Maxwell, C. E. Chase, and W. E. Millett, “Dielectric Constant of Liquid Helium,” in 5th International Conference on Low Temperature Physics and Chemistry, edited by J. R. Dillinger (University of Wisconsin Press, Madison, 1958), pp. 53-56. |
| 16 | C. E. Chase, E. Maxwell, and W. E. Millett, “The Dielectric Constant of Liquid Helium,” Physica 27, 1129-1145 (1961). |
| 17 | O. V. Lounsamaa and L. Kaunisto, “Direct Measurement of (dp/dt)v of Liquid Helium Near the Lambda-Curve,” in 7th International Conference on Low Temperature Physics, edited by G. M. Graham and A. C. Hollis Hallet (University of Toronto Press, Toronto, 1961), pp. 535-539. |
| 18 | E. C. Kerr and R. D. Taylor, “The Molar Volume and Expansion Coefficient of Liquid 4He,” Ann. Phys. 26, 292-306 (1964). |
| 19 | R. L. Mills and S. G. Sydoriak, “Thermal Expansion of Compressed Helium II,” Ann. Phys. 34, 276-290 (1965). |
| 20 | C. Boghosian and H. Meyer, “Density, Coefficient of Thermal Expansion and Entropy of Compression of Liquid 4He Under Pressure Below 1.4 K,” Phys. Rev. 152, 200-206 (1966). |
| 21 | V. P. Peshkov and A. P. Borovikov, “Measurement of the Lambda Transition Temperature and Density Maximum of Liquid 4He,” Sov. Phys. JETP 23, 559-565 (1966). |
| 22 | C. Boghosian and H. Meyer, “Density, Coefficient of Thermal Expansion, and Entropy Under Pressure below 1.4 K,” Phys. Rev. 163, 200-206 (1967). |
| 23 | D. L. Elwell and H. Meyer, “Molar Volume, Coefficient of Thermal Expansion and Related Properties of Liquid 4He Under Pressure,” Phys. Rev. 164, 245-255 (1967). |
| 24 | H. A. Kierstead, “Lambda Transformation of Liquid 4He at High Pressures,” Phys. Rev. 153, 258-262 (1967). |
| 25 | H. A. Kierstead, “Lambda Curve of Liquid 4He,” Phys. Rev. 162, 153-161 (1967). |
| 26 | P. R. Roach, “Pressure-Density-Temperature Surface of 4He Near the Critical Point,” Phys. Rev. 170, 213-223 (1968). |
| 27 | G. E. Watson, J. D. Reppy, and R. C. Richardson, “Low-Temperature Density and Soluability of 3He in Liquid 4He Under Pressure.,” Phys. Rev. 188, 384-396 (1969). |
| 28 | J. Wiebes, “Caloric Measurements on Liquid and Melting Helium Below 1.5 Kelvin,” Ph.D. Thesis, Kammerlingh Onnes Laboratory, 1969 (unpublished). |
| 29 | B. M. Abraham, H. Eckstein, J. B. Ketterson, M. Kuchnir, and P. R. Roach, “Velocity of Sound, Density, and Gruneisen Constant in Liquid 4He,” Phys. Rev. A 1, 250-257 (1970). |
| 30 | R. F. Harris-Lowe and K. A. Smee, “Thermal Expansion of Liquid Helium II,” Phys. Rev. A2, 158-161 (1970). |
| 31 | P. R. Roach, J. B. Ketterson, B. M. Abraham, and M. Kuchnir, “Thermal Expansion of Liquid 4He Between 0.1 and 0.7 K.,” Phys. Lett. A39, 251-252 (1972). |
| 32 | E. R. Grilly, “Pressure-Volume-Temperature Relations in Liquid and Solid 4He,” J. Low Temp. Phys. 11, 33-52 (1973) |
| 33 | C. T. Van Degrift, “Dielectric Constant, Density, and Expansion Coefficient of Liquid 4He at Vapor Pressure Below 4.4 K,” Ph.D. Thesis, University of California, 1974 (unpublished). |
| 34 | K. H. Mueller, F. Pobell, and G. Ahlers, “Thermal-Expansion Coefficient and Universality Near the Superfluid Transition of 4He Under Pressure,” Phys. Rev. Lett. 34, 513-516 (1975). |
| 35 | C. T. Van Degrift and J. R. Pellam, “Measurements of Temperature Dependence of Density of Liquid 4He from 0.3 K to 0.7 K and Near the Lambda-Point,” in 13th International Conference on Low Temperature Physics-LT13 (Plenum Press, NY-London, 1975), pp. 343-351. |
| 36 | J. E. Berthold, H. M. Hanson, H. J. Maris, and G. M. Seidel, “Investigation of the Phonon Dispersion Relation in Liquid 4He by Thermal-Expansion Measurements,” Phys. Rev. B14, 1902-1910 (1976). |
| 37 | H. N. Hanson, J. E. Berthold, G. M. Seidel, and H. J. Maris, “Density of Liquid 4He at Low Temperatures,” Phys. Rev. B14, 1911-1915 (1976). |
| 38 | H. A. Kierstead, “Dielectric Constant and Molar Volume of Saturated Liquid 3He and 4He,” J. Low Temp. Phys. 23, 791-805 (1976). |
| 39 | M. Chan, M. Ryschkewitsch, and H. Meyer, “The Dielectric Constant in Liquid and Solid 4He,” J. Low Temp. Phys. 26, 211-228 (1977). |
| 40 | J. J. Niemela and R. J. Donnelly, “Density and Thermal Expansion Coefficient of Liquid 4He from Measurements of the Dielectric Constant,” J. Low Temp. Phys. 98, 1-16 (1995) |
Chronological Bibliography for Dielectric Constant
1 |
M. Wolfke and W. H. Keesom, "On the Change of the Dielectric Constant of Liquid Helium with the Temperature. Provisional Measurements," Proc. Roy. Acad., Amsterdam 31, 81-94 (1928). |
| 2 | C. J. Grebenkamper and J. P. Hagen, “The Dielectric Constant of Liquid Helium,” Phys. Rev. 80, 89-91 (1950). |
| 3 | M. H. Edwards, “Refractive Index of 4He Liquid,” Can. J. Phys. 36, 884-898 (1958). |
| 4 | C. E. Chase, E. Maxwell, and W. E. Millett, “The Dielectric Constant of Liquid Helium,” Physica 27, 1129-1145 (1961). |
| 5 | M. J. Edwards and W. C. Woodbury, “Compressibility of Liquid 4He,” Can. J. Phys. 39, 1833-1841 (1961). |
| 6 | R. F. Harris-Lowe and K. A. Smee, “Thermal Expansion of Liquid Helium II,” Phys. Rev. A2, 158-161 (1970). |
| 7 | E. C. Kerr and H. N. Sherman, “The Molar Polarizability of 3He at Low Temperatures and Its Density Dependence,” J. Low Temp. Phys. 3, 451-461 (1970). |
| 8 | J. E. Berthold, H. M. Hanson, H. J. Maris, and G. M. Seidel, “Investigation of the Phonon Dispersion Relation in Liquid 4He by Thermal-Expansion Measurements,” Phys. Rev. B14, 1902-1910 (1976). |
| 9 | H. A. Kierstead, “Dielectric Constant and Molar Volume of Saturated Liquid 3He and 4He,” J. Low Temp. Phys. 23, 791-805 (1976). |
| 10 | M. Chan, M. Ryschkewitsch, and H. Meyer, “The Dielectric Constant in Liquid and Solid 4He,” J. Low Temp. Phys. 26, 211-228 (1977). |
| 11 | S. Wang, C. Howard, and H. Meyer, “Shear Viscosity of Liquid 4He and 3He-4He Mixtures, Especially Near the Superfluid Transition,” J. Low Temp. Phys. 26, 151-187 (1990). |
| 12 | J. J. Niemela and R. J. Donnelly, “Density and Thermal Expansion Coefficient of Liquid 4He from Measurements of the Dielectric Constant,” J. Low Temp. Phys. 98, 1-16 (1995 |
| <<Previous | Intro | ch1 | ch2 | ch3 | ch4 | ch5 | ch6 | ch7 | ch8 | ch9 | ch10 | Next>> |
| ch11 | ch12 | ch13 | ch14 | ch15 | ch16 | ch17 | ch18 | ch19 | ch20 | ch21 |

